Basic descriptive topics: | |
AN INTRODUCTION TO CHEMICAL EQUILIBRIA
It explains about at the basic ideas underpinning the idea of a chemical equilibrium. It talks about reversible reactions and how they behave if the system is closed. This leads to the idea of a dynamic equilibrium, and what the common term "position of equilibrium" means.
Reversible reactionsA reversible reaction is one which can be made to go in either direction depending on the conditions.If you pass steam over hot iron the steam reacts with the iron to produce a black, magnetic oxide of iron called triiron tetroxide, Fe3O4. The hydrogen produced in the reaction is swept away by the stream of steam. 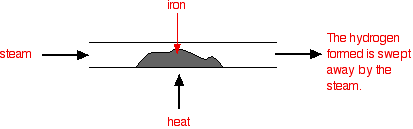 This time the steam produced in the reaction is swept away by the stream of hydrogen. 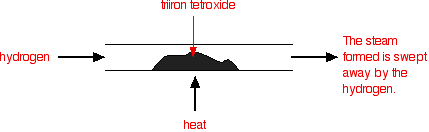 Reversible reactions happening in a closed system A closed system is one in which no substances are either added to the system or lost from it. Energy can, however, be transferred in or out at will. In the example we've been looking at, you would have to imagine iron being heated in steam in a closed container. Heat is being added to the system, but none of the substances in the reaction can escape. The system is closed. As the triiron tetroxide and hydrogen start to be formed, they will also react again to give the original iron and steam. So, if you analysed the mixture after a while, what would you find? You would find that you had established what is known as adynamic equilibrium. To explain what that means, we are going to use a much simpler example . . . Dynamic equilibriaGetting a visual feel for a dynamic equilibrium Imagine a substance which can exist in two forms - a blue form or an orange form - and that each form can react to give the other one. We are going to let them react in a closed system. Neither form can escape. Assume that the blue form turns into the orange one much faster than the other way round. In any given time, these are the chances of the two changes happening: 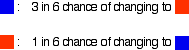 The following are the real results of a "reaction" I did myself. I started with 16 blue squares and looked at each one in turn and decided whether it should change colour by throwing a dice. A blue square was turned into an orange square (the bit of paper was turned over!) if I threw a 4, 5 or 6 An orange square was turned into a blue square only if I threw a 6 while I was looking at that particular square. Once I had looked at all 16 squares, I started the process all over again - but obviously with a different starting pattern. The diagrams show the results of doing this 11 times (plus the original 16 blue squares). 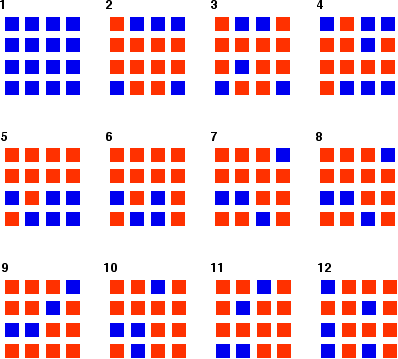 | |
Note: To be honest, this was a lucky fluke, given the small number of squares I was working with. If you repeated this with much larger number of squares (say, several thousand), you would find that your patterns settled down quite reliably close to 75% orange and 25% blue. On the other hand, that would be seriously tedious!If you had the huge numbers of particles taking part in chemical reactions, the proportions would be spot on 75% to 25%. | |
| Explaining the term "dynamic equilibrium" The reaction has reached equilibrium in the sense that there is no further change in the numbers of blue and orange squares. However, the reaction is still continuing. For every orange square that turns blue, somewhere in the mixture it is replaced by a blue square turning orange. This is known as a dynamic equilibrium. The word dynamicshows that the reaction is still continuing. You can show dynamic equilibrium in an equation for a reaction by the use of special arrows. In the present case, you would write it as: The "forward reaction" and the "back reaction" The change from left to right in the equation (in this case from blue to orange as it is written) is known as the forward reaction. The change from right to left is the back reaction. Position of equilibrium In the example we've used, the equilibrium mixture contained more orange squares than blue ones. Position of equilibrium is a way of expressing this. You can say things like:
For example, if changing the conditions produced more blue in the equilibrium mixture, you would say "The position of equilibrium has moved to the left" or "The position of equilibrium has moved towards the blue". | |
Note: If you can be bothered, try the effect on the position of equilibrium of increasing the chances of an orange square turning blue from 1 in 6 to 2 in 6. In other words, allow it to change if you throw either a 5 or a 6 with your dice. | |
| Reaching equilibrium from the other side What happens if you started the reaction with orange squares rather than blue ones, but kept the chances of each change happening the same as in the first example? This is the result of my "reaction". 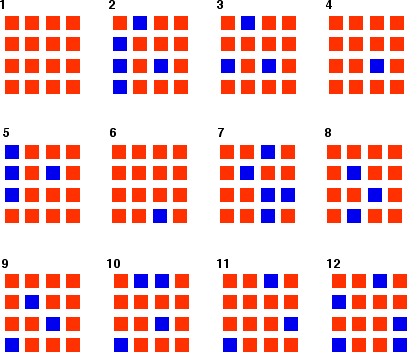 | |
Remember: You can't get the numbers to work out exactly using this small number of particles. Chance fluctuations are too noticeable. Once again, if you used really large numbers of particles, the equilibrium mixture would contain 75% orange and 25% blue. Given the number of particles we're working with, the "reaction" is remarkably close to that on average. | |
| A more formal look at dynamic equilibria Thinking about reaction rates This is the equation for a general reaction which has reached dynamic equilibrium: How did it get to that state? Let's assume that we started with A and B. At the beginning of the reaction, the concentrations of A and B were at their maximum. That means that the rate of the reaction was at its fastest. As A and B react, their concentrations fall. That means that they are less likely to collide and react, and so the rate of the forward reaction falls as time goes on. 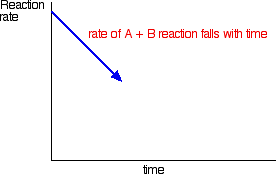 With time, the rate of the reaction between C and D increases: 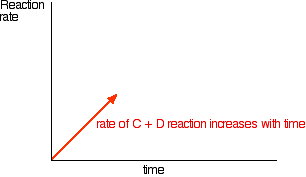 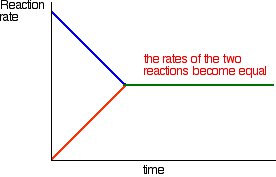 | |
LE CHATELIER'S PRINCIPLE
It explains Le Chatelier's Principle and explains how to apply it to reactions in a state of dynamic equilibrium. It covers changes to the position of equilibrium if you change concentration, pressure or temperature. It also explains very briefly why catalysts have no effect on the position of equilibrium.
| |
It is important in understanding everything on this page to realise that Le Chatelier's Principle is no more than a useful guide to help you work out what happens when you change the conditions in a reaction in dynamic equilibrium. It doesn't explain anything. I'll keep coming back to that point!
Using Le Chatelier's Principle
A statement of Le Chatelier's Principle
Using Le Chatelier's Principle with a change of concentration
Suppose you have an equilibrium established between four substances A, B, C and D.
| |
What would happen if you changed the conditions by increasing the concentration of A?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the concentration of A decreases again - by reacting it with B and turning it into C + D. The position of equilibrium moves to the right.

This is a useful way of converting the maximum possible amount of B into C and D. You might use it if, for example, B was a relatively expensive material whereas A was cheap and plentiful.
What would happen if you changed the conditions by decreasing the concentration of A?
According to Le Chatelier, the position of equilibrium will move so that the concentration of A increases again. That means that more C and D will react to replace the A that has been removed. The position of equilibrium moves to the left.

This is esssentially what happens if you remove one of the products of the reaction as soon as it is formed. If, for example, you removed C as soon as it was formed, the position of equilibrium would move to the right to replace it. If you kept on removing it, the equilibrium position would keep on moving rightwards - turning this into a one-way reaction.
| |
Using Le Chatelier's Principle with a change of pressure
This only applies to reactions involving gases:
What would happen if you changed the conditions by increasing the pressure?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the pressure is reduced again.
Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules.
In this case, there are 3 molecules on the left-hand side of the equation, but only 2 on the right. By forming more C and D, the system causes the pressure to reduce.
Increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer molecules.

What would happen if you changed the conditions by decreasing the pressure?
The equilibrium will move in such a way that the pressure increases again. It can do that by producing more molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.

What happens if there are the same number of molecules on both sides of the equilibrium reaction?
In this case, increasing the pressure has no effect whatsoever on the position of the equilibrium. Because you have the same numbers of molecules on both sides, the equilibrium can't move in any way that will reduce the pressure again.
| |
Using Le Chatelier's Principle with a change of temperature
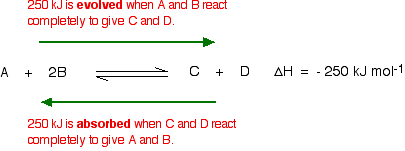
For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved):
This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A reacts completely with 2 moles of B. For reversible reactions, the value is always given as if the reaction was one-way in the forward direction.
The back reaction (the conversion of C and D into A and B) would be endothermic by exactly the same amount.
What would happen if you changed the conditions by increasing the temperature?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the temperature is reduced again.
Suppose the system is in equilibrium at 300°C, and you increase the temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again?
To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reaction absorbs heat. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D.
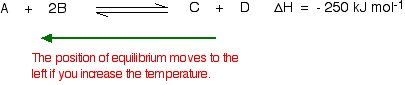
If you were aiming to make as much C and D as possible, increasing the temperature on a reversible reaction where the forward reaction is exothermic isn't a good idea!
What would happen if you changed the conditions by decreasing the temperature?
The equilibrium will move in such a way that the temperature increases again.
Suppose the system is in equilibrium at 500°C and you reduce the temperature to 400°C. The reaction will tend to heat itself up again to return to the original temperature. It can do that by favouring the exothermic reaction.
The position of equilibrium will move to the right. More A and B are converted into C and D at the lower temperature.
 | |
Examples:
THE HABER PROCESS
It describes the Haber Process for the manufacture of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. It looks at the effect of temperature, pressure and catalyst on the composition of the equilibrium mixture, the rate of the reaction and the economics of the process.
A brief summary of the Haber Process
The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The reaction is reversible and the production of ammonia is exothermic.
A flow scheme for the Haber Process looks like this:
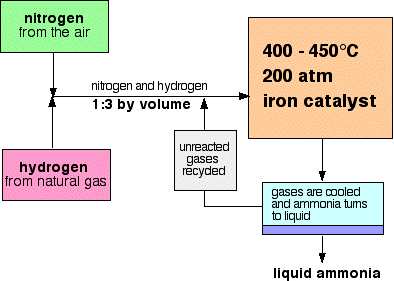
Some notes on the conditions
The catalyst
The catalyst is actually slightly more complicated than pure iron. It has potassium hydroxide added to it as a promoter - a substance that increases its efficiency.
The pressure
The pressure varies from one manufacturing plant to another, but is always high. You can't go far wrong in an exam quoting 200 atmospheres.
Recycling
At each pass of the gases through the reactor, only about 15% of the nitrogen and hydrogen converts to ammonia. (This figure also varies from plant to plant.) By continual recycling of the unreacted nitrogen and hydrogen, the overall conversion is about 98%.
Explaining the conditions
The proportions of nitrogen and hydrogen
The mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen.
Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means that the gases are going into the reactor in the ratio of 1 molecule of nitrogen to 3 of hydrogen.
That is the proportion demanded by the equation.
In some reactions you might choose to use an excess of one of the reactants. You would do this if it is particularly important to use up as much as possible of the other reactant - if, for example, it was much more expensive. That doesn't apply in this case.
There is always a down-side to using anything other than the equation proportions. If you have an excess of one reactant there will be molecules passing through the reactor which can't possibly react because there isn't anything for them to react with. This wastes reactor space - particularly space on the surface of the catalyst.
The temperature
Equilibrium considerations
You need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of ammonia in the equilibrium mixture.
The forward reaction (the production of ammonia) is exothermic.
According to Le Chatelier's Principle, this will be favoured if you lower the temperature. The system will respond by moving the position of equilibrium to counteract this - in other words by producing more heat.
In order to get as much ammonia as possible in the equilibrium mixture, you need as low a temperature as possible. However, 400 - 450°C isn't a low temperature!
Rate considerations
The lower the temperature you use, the slower the reaction becomes. A manufacturer is trying to produce as much ammonia as possible per day. It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of ammonia if it takes several years for the reaction to reach that equilibrium.
You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor.
The compromise
400 - 450°C is a compromise temperature producing a reasonably high proportion of ammonia in the equilibrium mixture (even if it is only 15%), but in a very short time.
The pressure
Equilibrium considerations
Notice that there are 4 molecules on the left-hand side of the equation, but only 2 on the right.
According to Le Chatelier's Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again.
In order to get as much ammonia as possible in the equilibrium mixture, you need as high a pressure as possible. 200 atmospheres is a high pressure, but not amazingly high.
Rate considerations
Increasing the pressure brings the molecules closer together. In this particular instance, it will increase their chances of hitting and sticking to the surface of the catalyst where they can react. The higher the pressure the better in terms of the rate of a gas reaction.
Economic considerations
Very high pressures are very expensive to produce on two counts.
You have to build extremely strong pipes and containment vessels to withstand the very high pressure. That increases your capital costs when the plant is built.
High pressures cost a lot to produce and maintain. That means that the running costs of your plant are very high.
The compromise
200 atmospheres is a compromise pressure chosen on economic grounds. If the pressure used is too high, the cost of generating it exceeds the price you can get for the extra ammonia produced.
The catalyst
Equilibrium considerations
The catalyst has no effect whatsoever on the position of the equilibrium. Adding a catalyst doesn't produce any greater percentage of ammonia in the equilibrium mixture. Its only function is to speed up the reaction.
Rate considerations
In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor.
Separating the ammonia
When the gases leave the reactor they are hot and at a very high pressure. Ammonia is easily liquefied under pressure as long as it isn't too hot, and so the temperature of the mixture is lowered enough for the ammonia to turn to a liquid. The nitrogen and hydrogen remain as gases even under these high pressures, and can be recycled.
THE CONTACT PROCESS
It describes the Contact Process for the manufacture of sulphuric acid, and then goes on to explain the reasons for the conditions used in the process. It looks at the effect of proportions, temperature, pressure and catalyst on the composition of the equilibrium mixture, the rate of the reaction and the economics of the process.
| |
A brief summary of the Contact Process
The Contact Process:
- makes sulphur dioxide;
- convers the sulphur dioxide into sulphur trioxide (the reversible reaction at the heart of the process);
- converts the sulphur trioxide into concentrated sulphuric acid.
Making the sulphur dioxide
This can either be made by burning sulphur in an excess of air:
. . . or by heating sulphide ores like pyrite in an excess of air:
In either case, an excess of air is used so that the sulphur dioxide produced is already mixed with oxygen for the next stage.
Converting the sulphur dioxide into sulphur trioxide
This is a reversible reaction, and the formation of the sulphur trioxide is exothermic.
A flow scheme for this part of the process looks like this:
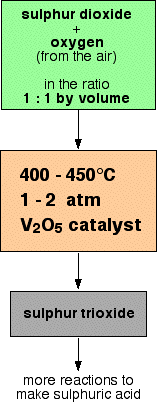
The reasons for all these conditions will be explored in detail further down the page.
Converting the sulphur trioxide into sulphuric acid
This can't be done by simply adding water to the sulphur trioxide - the reaction is so uncontrollable that it creates a fog of sulphuric acid. Instead, the sulphur trioxide is first dissolved in concentrated sulphuric acid:
The product is known as fuming sulphuric acid or oleum.
This can then be reacted safely with water to produce concentrated sulphuric acid - twice as much as you originally used to make the fuming sulphuric acid.
Explaining the conditions
The proportions of sulphur dioxide and oxygen
The mixture of sulphur dioxide and oxygen going into the reactor is in equal proportions by volume.
Avogadro's Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means that the gases are going into the reactor in the ratio of 1 molecule of sulphur dioxide to 1 of oxygen.
That is an excess of oxygen relative to the proportions demanded by the equation.
According to Le Chatelier's Principle, Increasing the concentration of oxygen in the mixture causes the position of equilibrium to shift towards the right. Since the oxygen comes from the air, this is a very cheap way of increasing the conversion of sulphur dioxide into sulphur trioxide.
Why not use an even higher proportion of oxygen? This is easy to see if you take an extreme case. Suppose you have a million molecules of oxygen to every molecule of sulphur dioxide.
The equilibrium is going to be tipped very strongly towards sulphur trioxide - virtually every molecule of sulphur dioxide will be converted into sulphur trioxide. Great! But you aren't going to produce much sulphur trioxide every day. The vast majority of what you are passing over the catalyst is oxygen which has nothing to react with.
By increasing the proportion of oxygen you can increase the percentage of the sulphur dioxide converted, but at the same time decrease the total amount of sulphur trioxide made each day. The 1 : 1 mixture turns out to give you the best possible overall yield of sulphur trioxide.
The temperature
Equilibrium considerations
You need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of sulphur trioxide in the equilibrium mixture.
The forward reaction (the production of sulphur trioxide) is exothermic.
According to Le Chatelier's Principle, this will be favoured if you lower the temperature. The system will respond by moving the position of equilibrium to counteract this - in other words by producing more heat.
In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as low a temperature as possible. However, 400 - 450°C isn't a low temperature!
Rate considerations
The lower the temperature you use, the slower the reaction becomes. A manufacturer is trying to produce as much sulphur trioxide as possible per day. It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of sulphur trioxide if it takes several years for the reaction to reach that equilibrium.
You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor.
The compromise
400 - 450°C is a compromise temperature producing a fairly high proportion of sulphur trioxide in the equilibrium mixture, but in a very short time.
The pressure
Equilibrium considerations
Notice that there are 3 molecules on the left-hand side of the equation, but only 2 on the right.
According to Le Chatelier's Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again.
In order to get as much sulphur trioxide as possible in the equilibrium mixture, you need as high a pressure as possible. High pressures also increase the rate of the reaction. However, the reaction is done at pressures close to atmospheric pressure!
Economic considerations
Even at these relatively low pressures, there is a 99.5% conversion of sulphur dioxide into sulphur trioxide. The very small improvement that you could achieve by increasing the pressure isn't worth the expense of producing those high pressures.
The catalyst
Equilibrium considerations
The catalyst has no effect whatsoever on the position of the equilibrium. Adding a catalyst doesn't produce any greater percentage of sulphur trioxide in the equilibrium mixture. Its only function is to speed up the reaction.
Rate considerations
In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor.
Mathematical bits:
EQUILIBRIUM CONSTANTS: Kc
It explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, Kc. It assumes that you are familiar with the concept of a dynamic equilibrium, and know what is meant by the terms "homogeneous" and "heterogeneous" as applied to chemical reactions.
We need to look at two different types of equilibria (homogeneous and heterogeneous) separately, because the equilibrium constants are defined differently.
- A homogeneous equilibrium has everything present in the same phase. The usual examples include reactions where everything is a gas, or everything is present in the same solution.
- A heterogeneous equilibrium has things present in more than one phase. The usual examples include reactions involving solids and gases, or solids and liquids.
Kc in homogeneous equilibria
This is the more straightforward case. It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same solution.
A good example of a gaseous homogeneous equilibrium is the conversion of sulphur dioxide to sulphur trioxide at the heart of the Contact Process:
A commonly used liquid example is the esterification reaction between an organic acid and an alcohol - for example:
Writing an expression for Kc
We are going to look at a general case with the equation:
No state symbols have been given, but they will be all (g), or all (l), or all (aq) if the reaction was between substances in solution in water.
If you allow this reaction to reach equilibrium and then measure the equilibrium concentrations of everything, you can combine these concentrations into an expression known as an equilibrium constant.
The equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of A, B, C and D you started with. It is also unaffected by a change in pressure or whether or not you are using a catalyst.
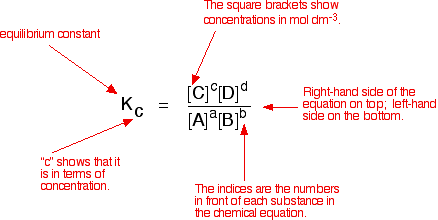
Compare this with the chemical equation for the equilibrium. The convention is that the substances on the right-hand side of the equation are written at the top of the Kc expression, and those on the left-hand side at the bottom.
The indices (the powers that you have to raise the concentrations to - for example, squared or cubed or whatever) are just the numbers that appear in the equation.
Some specific examples
The esterification reaction equilibrium
A typical equation might be:
There is only one molecule of everything shown in the equation. That means that all the powers in the equilibrium constant expression are "1". You don't need to write those into the Kcexpression.
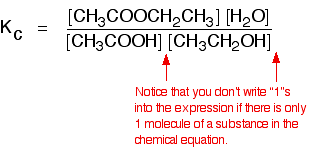
As long as you keep the temperature the same, whatever proportions of acid and alcohol you mix together, once equilibrium is reached, Kc always has the same value. At room temperature, this value is approximately 4 for this reaction.
The equilibrium in the hydrolysis of esters
This is the reverse of the last reaction:
The Kc expression is:
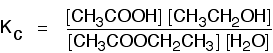
If you compare this with the previous example, you will see that all that has happened is that the expression has turned upside-down. Its value at room temperature will be approximately 1/4 (0.25).
It is really important to write down the equilibrium reaction whenever you talk about an equilibrium constant. That is the only way that you can be sure that you have got the expression the right way up - with the right-hand substances on the top and the left-hand ones at the bottom.
The Contact Process equilibrium
You will remember that the equation for this is:
This time the Kc expression will include some visible powers:
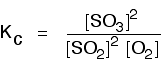
Although everything is present as a gas, you still measure concentrations in mol dm-3. There is another equilibrium constant called Kp which is more frequently used for gases. You will find a link to that at the bottom of the page.
The Haber Process equilibrium
The equation for this is:
. . . and the Kc expression is:
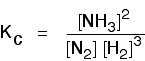
Kc in heterogeneous equilibria
Typical examples of a heterogeneous equilibrium include:
The equilibrium established if steam is in contact with red hot carbon. Here we have gases in contact with a solid.
If you shake copper with silver nitrate solution, you get this equilibrium involving solids and aqueous ions:
Writing an expression for Kc for a heterogeneous equilibrium
The important difference this time is that you don't include any term for a solid in the equilibrium expression.
Taking another look at the two examples above, and adding a third one:
The equilibrium produced on heating carbon with steam
Everything is exactly the same as before in the equilibrium constant expression, except that you leave out the solid carbon.
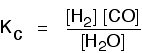
The equilibrium produced between copper and silver ions
Both the copper on the left-hand side and the silver on the right are solids. Both are left out of the equilibrium constant expression.
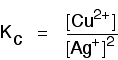
The equilibrium produced on heating calcium carbonate
This equilibrium is only established if the calcium carbonate is heated in a closed system, preventing the carbon dioxide from escaping.
The only thing in this equilibrium which isn't a solid is the carbon dioxide. That is all that is left in the equilibrium constant expression.
Calculations involving Kc
There are all sorts of calculations you might be expected to do which are centred around equilibrium constants. You might be expected to calculate a value for Kc including its units (which vary from case to case). Alternatively you might have to calculate equilibrium concentrations from a given value of Kc and given starting concentrations.
This is simply too huge a topic to be able to deal with satisfactorily on the internet. It isn't the best medium for learning how to do chemistry calculations. It is much easier to do this from a carefully structured book giving you lots of worked examples and lots of problems to try yourself.
If you have found this site useful, you might like to have a look at my book on chemistry calculations. It covers equilibrium constant calculations starting with the most trivial cases, and gradually getting harder - up to the moderately difficult examples which may be asked in a UK A' level examination.
EQUILIBRIUM CONSTANTS: Kp
It explains equilibrium constants expressed in terms of partial pressures of gases, Kp. It covers an explanation of the terms mole fraction and partial pressure, and looks at Kp for both homogeneous and heterogeneous reactions involving gases.
The page assumes that you are already familiar with the concept of an equilibrium constant, and that you know about Kc - an equilibrium constant expressed in terms of concentrations
Defining some terms
Before we can go any further, there are two terms relating to mixtures of gases that you need to be familiar with.
Mole fraction
If you have a mixture of gases (A, B, C, etc), then the mole fraction of gas A is worked out by dividing the number of moles of A by the total number of moles of gas.
The mole fraction of gas A is often given the symbol xA. The mole fraction of gas B would be xB - and so on.
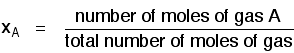
Pretty obvious really!
For example, in a mixture of 1 mole of nitrogen and 3 moles of hydrogen, there are a total of 4 moles of gas. The mole fraction of nitrogen is 1/4 (0.25) and of hydrogen is 3/4 (0.75).
Partial pressure
The partial pressure of one of the gases in a mixture is the pressure which it would exert if it alone occupied the whole container.
The partial pressure of gas A is often given the symbol PA. The partial pressure of gas B would be PB - and so on.
There are two important relationships involving partial pressures. The first is again fairly obvious.
The total pressure of a mixture of gases is equal to the sum of the partial pressures.
It is easy to see this visually:
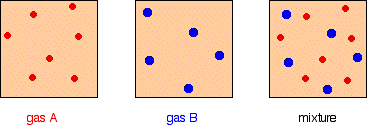
Gas A is creating a pressure (its partial pressure) when its molecules hit the walls of its container. Gas B does the same. When you mix them up, they just go on doing what they were doing before. The total pressure is due to both molecules hitting the walls - in other words, the sum of the partial pressures.
The more important relationship is the second one:
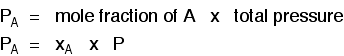
Learn it!
That means that if you had a mixture made up of 20 moles of nitrogen, 60 moles of hydrogen and 20 moles of ammonia (a total of 100 moles of gases) at 200 atmospheres pressure, the partial pressures would be calculated like this:
| gas | mole fraction | partial pressure |
|---|---|---|
| nitrogen | 20/100 = 0.2 | 0.2 x 200 = 40 atm |
| hydrogen | 60/100 = 0.6 | 0.6 x 200 = 120 atm |
| ammonia | 20/100 = 0.2 | 0.2 x 200 = 40 atm |
Partial pressures can be quoted in any normal pressure units. The common ones are atmospheres or pascals (Pa). Pascals are exactly the same as N m-2 (newtons per square metre).
Kp in homogeneous gaseous equilibria
A homogeneous equilibrium is one in which everything in the equilibrium mixture is present in the same phase. In this case, to use Kp, everything must be a gas.
A good example of a gaseous homogeneous equilibrium is the conversion of sulphur dioxide to sulphur trioxide at the heart of the Contact Process:
Writing an expression for Kp
We are going to start by looking at a general case with the equation:
If you allow this reaction to reach equilibrium and then measure (or work out) the equilibrium partial pressures of everything, you can combine these into the equilibrium constant, Kp.
Just like Kc, Kp always has the same value (provided you don't change the temperature), irrespective of the amounts of A, B, C and D you started with.
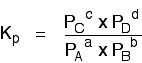
Kp has exactly the same format as Kc, except that partial pressures are used instead of concentrations. The gases on the right-hand side of the chemical equation are at the top of the expression, and those on the left at the bottom.
The Contact Process equilibrium
You will remember that the equation for this is:
Kp is given by:
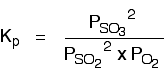
The Haber Process equilibrium
The equation for this is:
. . . and the Kp expression is:
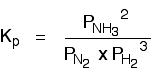
Kp in heterogeneous equilibria
A typical example of a heterogeneous equilibrium will involve gases in contact with solids.
Writing an expression for Kp for a heterogeneous equilibrium
Exactly as happens with Kc, you don't include any term for a solid in the equilibrium expression.
The next two examples have already appeared on the Kc page.
The equilibrium produced on heating carbon with steam
Everything is exactly the same as before in the expression for Kp, except that you leave out the solid carbon.
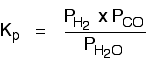
The equilibrium produced on heating calcium carbonate
This equilibrium is only established if the calcium carbonate is heated in a closed system, preventing the carbon dioxide from escaping.
The only thing in this equilibrium which isn't a solid is the carbon dioxide. That is all that is left in the equilibrium constant expression.
Calculations involving Kp
On the Kc page, I've already discussed the fact that the internet isn't a good medium for learning how to do calculations.
If you want lots of worked examples and problems to do yourself centred around Kp, you might be interested in my book on chemistry calculations.
EQUILIBRIUM CONSTANTS and LE CHATELIER'S PRINCIPLE
It shows the relationship between equilibrium constants and Le Chatelier's Principle. Students often get confused about how it is possible for the position of equilibrium to change as you change the conditions of a reaction, although the equilibrium constant may remain the same.
Be warned that this page assumes a good understanding of Le Chatelier's Principle and how to write expressions for equilibrium constants.
| Changing concentrationsThe facts Equilibrium constants aren't changed if you change the concentrations of things present in the equilibrium. The only thing that changes an equilibrium constant is a change of temperature. The position of equilibrium is changed if you change the concentration of something present in the mixture. According to Le Chatelier's Principle, the position of equilibrium moves in such a way as to tend to undo the change that you have made. Suppose you have an equilibrium established between four substances A, B, C and D. According to Le Chatelier's Principle, if you decrease the concentration of C, for example, the position of equilibrium will move to the right to increase the concentration again. | |
Explanation in terms of the constancy of the equilibrium constant
The equilibrium constant, Kc for this reaction looks like this:
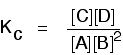
If you have moved the position of the equilibrium to the right (and so increased the amount of C and D), why hasn't the equilibrium constant increased?
This is actually the wrong question to ask! We need to look at it the other way round.
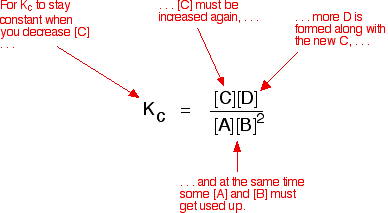
Let's assume that the equilibrium constant mustn't change if you decrease the concentration of C - because equilibrium constants are constant at constant temperature. Why does the position of equilibrium move as it does?
If you decrease the concentration of C, the top of the Kcexpression gets smaller. That would change the value of Kc. In order for that not to happen, the concentrations of C and D will have to increase again, and those of A and B must decrease. That happens until a new balance is reached when the value of the equilibrium constant expression reverts to what it was before.
The position of equilibrium moves - not because Le Chatelier says it must - but because of the need to keep a constant value for the equilibrium constant.
If you decrease the concentration of C:
Changing pressure
This only applies to systems involving at least one gas.
The facts
Equilibrium constants aren't changed if you change the pressure of the system. The only thing that changes an equilibrium constant is a change of temperature.
The position of equilibrium may be changed if you change the pressure. According to Le Chatelier's Principle, the position of equilibrium moves in such a way as to tend to undo the change that you have made.
That means that if you increase the pressure, the position of equilibrium will move in such a way as to decrease the pressure again - if that is possible. It can do this by favouring the reaction which produces the fewer molecules. If there are the same number of molecules on each side of the equation, then a change of pressure makes no difference to the position of equilibrium.
Explanation
Where there are different numbers of molecules on each side of the equation
Let's look at the same equilibrium we've used before. This one would be affected by pressure because there are 3 molecules on the left but only 2 on the right. An increase in pressure would move the position of equilibrium to the right.
Because this is an all-gas equilibriium, it is much easier to use Kp:
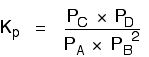
Once again, it is easy to suppose that, because the position of equilibrium will move to the right if you increase the pressure, Kpwill increase as well. Not so!
To understand why, you need to modify the Kp expression.
Remember the relationship between partial pressure, mole fraction and total pressure?

Replacing all the partial pressure terms by mole fractions and total pressure gives you this:
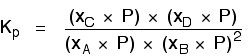
If you sort this out, most of the "P"s cancel out - but one is left at the bottom of the expression.
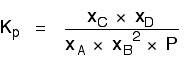
Now, remember that Kp has got to stay constant because the temperature is unchanged. How can that happen if you increase P?
To compensate, you would have to increase the terms on the top, xC and xD, and decrease the terms on the bottom, xA and xB.
Increasing the terms on the top means that you have increased the mole fractions of the molecules on the right-hand side. Decreasing the terms on the bottom means that you have decreased the mole fractions of the molecules on the left.
That is another way of saying that the position of equilibrium has moved to the right - exactly what Le Chatelier's Principle predicts. The position of equilibrium moves so that the value of Kp is kept constant.
Where there are the same numbers of molecules on each side of the equation
In this case, the position of equilibrium isn't affected by a change of pressure. Why not?
Let's go through the same process as before:
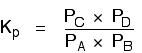
Substituting mole fractions and total pressure:
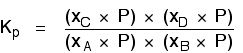
. . . and cancelling out as far as possible:
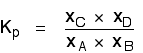
There isn't a single "P" left in the expression. Changing the pressure can't make any difference to the Kp expression. The position of equilibrium doesn't need to move to keep Kp constant.
Changing temperature
The facts
Equilibrium constants are changed if you change the temperature of the system. Kc or Kp are constant at constant temperature, but they vary as the temperature changes.
Look at the equilibrium involving hydrogen, iodine and hydrogen iodide:
The Kp expression is:
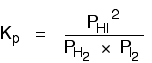
Two values for Kp are:
| temperature | Kp |
|---|---|
| 500 K | 160 |
| 700 K | 54 |
You can see that as the temperature increases, the value of Kpfalls.
| This is typical of what happens with any equilibrium where the forward reaction is exothermic. Increasing the temperature decreases the value of the equilibrium constant. Where the forward reaction is endothermic, increasing the temperature increases the value of the equilibrium constant. | |
Note: Any explanation for this needs knowledge beyond the scope of any UK A level (or equivalent) syllabus. | |
| The position of equilibrium also changes if you change the temperature. According to Le Chatelier's Principle, the position of equilibrium moves in such a way as to tend to undo the change that you have made. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. It will do that by favouring the reaction which absorbs heat. In the equilibrium we've just looked at, that will be the back reaction because the forward reaction is exothermic. So, according to Le Chatelier's Principle the position of equilibrium will move to the left. Less hydrogen iodide will be formed, and the equilibrium mixture will contain more unreacted hydrogen and iodine. That is entirely consistent with a fall in the value of the equilibrium constant. 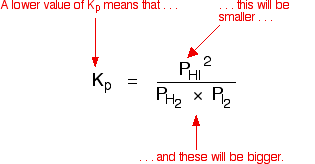 Equilibrium constants aren't changed if you add (or change) a catalyst. The only thing that changes an equilibrium constant is a change of temperature. The position of equilibrium is not changed if you add (or change) a catalyst. Explanation A catalyst speeds up both the forward and back reactions by exactly the same amount. Dynamic equilibrium is established when the rates of the forward and back reactions become equal. If a catalyst speeds up both reactions to the same extent, then they will remain equal without any need for a shift in position of equilibrium. | |
No comments:
Post a Comment