Introduction
Fluid Mechanicsis that section of applied mechanics, concerned with the statics and dynamics of liquids and gases.
A knowledge of fluid mechanics is essential for the chemical engineer, because the majority of chemical processing operations are conducted either partially or totally in the fluid phase.
The handling of liquids is much simpler, much cheaper, and much less troublesome than handling solids.
Even in many operations a solid is handled in a finely divided state so that it stays in suspension in a fluid.
Fluid Statics: Which treats fluids in the equilibrium state of no shear stress
Fluid Mechanics: Which treats when portions of fluid are in motion relative to other parts.
In everyday life, we recognize three states of matter:solid,liquid and gas. Although different in many respects, liquids and gases have a common characteristic in which they differ from solids: they are fluids, lacking the ability of solids to offer a permanent resistance to a deforming force.
A fluid is a substance which deforms continuously under the action of shearing forces, however small they may be.Conversely, it follows that:
If a fluid is at rest, there can be no shearing forces acting and, therefore, all forces in the fluid must be perpendicular to the planes upon which they act.
If a fluid is at rest, there can be no shearing forces acting and, therefore, all forces in the fluid must be perpendicular to the planes upon which they act.
Shear stress in a moving fluid
Although there can be no shear stress in a fluid at rest, shear stresses are developed when the fluid is in motion, if the particles of the fluid move relative to each other so that they have different velocities, causing the original shape of the fluid to become distorted. If, on the other hand, the velocity of the fluid is same at every point, no shear stresses will be produced, since the fluid particles are at rest relative to each other.
Differences between solids and fluids:
The differences between the behaviours of solids and fluids under an applied force are as follows:
- For a solid, the strain is a function of the applied stress, providing that the elastic limit is not exceeded. For a fluid, the rate of strain is proportional to the applied stress.
- The strain in a solid is independent of the time over which the force is applied and, if the elastic limit is not exceeded, the deformation disappears when the force is removed. A fluid continues to flow as long as the force is applied and will not recover its original form when the force is removed.
Differences between liquids and gases:
Although liquids and gases both share the common characteristics of fluids, they have many distinctive characteristics of their own. A liquid is difficult to compress and, for many purposes, may be regarded as incompressible. A given mass of liquid occupies a fixed volume, irrespective of the size or shape of its container, and a free surface is formed if the volume of the container is greater than that of the liquid.
A gas is comparatively easy to compress. Changes of volume with pressure are large, cannot normally be neglected and are related to changes of temperature. A given mass of gas has no fixed volume and will expand continuously unless restrained by a containing vessel. It will completely fill any vessel in which it is placed and, therefore, does not form a free surface.
Types of Fluids:
Newtonian and non-Newtonian Fluids
Newtonian fluids:
Fluids which obey the Newton's law of viscosity are called as Newtonian fluids. Newton's law of viscosity is given by
t = mdv/dy
where t = shear stress
m = viscosity of fluid
dv/dy = shear rate, rate of strain or velocity gradient
All gases and most liquids which have simpler molecular formula and low molecular weight such as water, benzene, ethyl alcohol, CCl4, hexane and most solutions of simple molecules are Newtonian fluids.
Non-Newtonian fluids:
Fluids which do not obey the Newton's law of viscosity are called as non-Newtonian fluids.
Generally non-Newtonian fluids are complex mixtures: slurries, pastes, gels, polymer solutions etc.,
Various non-Newtonian Behaviors:
Properties are independent of time under shear.
Bingham-plastic: Resist a small shear stress but flow easily under larger shear stresses. e.g. tooth-paste, jellies, and some slurries.
Pseudo-plastic: Most non-Newtonian fluids fall into this group. Viscosity decreases with increasing velocity gradient. e.g. polymer solutions, blood. Pseudoplastic fluids are also called as Shear thinning fluids. At low shear rates(du/dy) the shear thinning fluid is more viscous than the Newtonian fluid, and at high shear rates it is less viscous.
Dilatant fluids: Viscosity increases with increasing velocity gradient. They are uncommon, but suspensions of starch and sand behave in this way. Dilatant fluids are also called as shear thickening fluids.
Time dependent behaviors:
Those which are dependent upon duration of shear.
Thixotropic fluids: for which the dynamic viscosity decreases with the time for which shearing forces are applied. e.g. thixotropic jelly paints.
Rheopectic fluids: Dynamic viscosity increases with the time for which shearing forces are applied. e.g. gypsum suspension in water.
Visco-elastic fluids: Some fluids have elastic properties, which allow them to spring back when a shear force is released. e.g. egg white.
Physical properties:
The viscosity (m) of a fluid measures its resistance to flow under an applied shear stress. Representative units for viscosity are kg/(m.sec), g/(cm.sec) (also known as poise designated by P). The centipoise (cP), one hundredth of a poise, is also a convenient unit, since the viscosity of water at room temperature is approximately 1 centipoise.
The kinematic viscosity (n) is the ratio of the viscosity to the density:
n = m/r,
and will be found to be important in cases in which significant viscous and gravitational forces exist.
Viscosity of liquids:
Viscosity of liquids in general, decreases with increasing temperature.
The viscosities (m) of liquids generally vary approximately with absolute temperature T according to:
ln m = a - b ln T
Viscosity of gases:Viscosity of gases increases with increase in temperature.The viscosity (m) of many gases is approximated by the formula:
m = mo(T/To)n
in which T is the absolute temperature, mo is the viscosity at an absolute reference temperature To, and n is an empirical exponent that best fits the experimental data.
The viscosity of an ideal gas is independent of pressure, but the viscosities of real gases and liquids usually increase with pressure.Viscosity of liquids are generally two orders of magnitude greater than gases at atmospheric pressure. Fow example, at 25oC, mwater = 1 centipoise and mair = 1 x 10-2centipoise.
Vapor Pressure
The pressure at which a liquid will boil is called its vapor pressure. This pressure is a function of temperature (vapor pressure increases with temperature). In this context we usually think about the temperature at which boiling occurs. For example, water boils at 100oC at sea-level atmospheric pressure (1 atm abs). However, in terms of vapor pressure, we can say that by increasing the temperature of water at sea level to 100 oC, we increase the vapor pressure to the point at which it is equal to the atmospheric pressure (1 atm abs), so that boiling occurs. It is easy to visualize that boiling can also occur in water at temperatures much below 100oC if the pressure in the water is reduced to its vapor pressure. For example, the vapor pressure of water at 10oC is 0.01 atm. Therefore, if the pressure within water at that temperature is reduced to that value, the water boils. Such boiling often occurs in flowing liquids, such as on the suction side of a pump. When such boiling does occur in the flowing liquids, vapor bubbles start growing in local regions of very low pressre and then collapse in regions of high downstream pressure. This phenomenon is called as cavitation.
Compressibility and the Bulk modulus:
All materials, whether solids, liquids or gases, are compressible, i.e. the volume V of a given mass will be reduced to V - dV when a force is exerted uniformly all over its surface. If the force per unit area of surface increases from p to p + dp, the relationship between change of pressure and change of volume depends on the bulk modulus of the material.
Bulk modulus (K) = (change in pressure) / (volumetric strain)
Volumetric strain is the change in volume divided by the original volume. Therefore,
(change in volume) / (original volume) = (change in pressure) / (bulk modulus)
i.e., -dV/V = dp/K
Negative sign for dV indicates the volume decreases as pressure increases.
In the limit, as dp tends to 0,
K = -V dp/dV à 1
Considering unit mass of substance, V = 1/r à 2
Differentiating,
Vdr + rdV = 0
dV = - (V/r)dr à3
putting the value of dV from equn.3 to equn.1,
K = - V dp / (-(V/r)dr)
i.e. K = rdp/dr
The concept of the bulk modulus is mainly applied to liquids, since for gases the compressibility is so great that the value of K is not a constant.
The relationship between pressure and mass density is more conveniently found from the characteristic equation of gas.
For liquids, the changes in pressure occurring in many fluid mechanics problems are not sufficiently great to cause appreciable changes in density. It is therefore usual to ignore such changes and consider liquids as incompressible.
Gases may also be treated as incompressible if the pressure changes are very small, but usually compressibility cannot be ignored. In general, compressibility becomes important when the velocity of the fluid exceeds about one-fifth of the velocity of a pressure wave (velocity of sound) in the fluid.
Typical values of Bulk Modulus:
K = 2.05 x 109 N/m2 for water
K = 1.62 x 109 N/m2 for oil.
Surface Tension
A molecule I in the interior of a liquid is under attractive forces in all directions and the vector sum of these forces is zero. But a molecule S at the surface of a liquid is acted by a net inward cohesive force that is perpendicular to the surface. Hence it requires work to move molecules to the surface against this opposing force, and surface molecules have more energy than interior ones.
The surface tension (s sigma) of a liquid is the work that must be done to bring enough molecules from inside the liquid to the surface to form one new unit area of that surface (J/m2 = N/m). Historically surface tensions have been reported in handbooks in dynes per centimeter (1 dyn/cm = 0.001 N/m).
Surface tension is the tendency of the surface of a liquid to behave like a stretched elastic membrane. There is a natural tendency for liquids to minimize their surface area. For this reason, drops of liquid tend to take a spherical shape in order to minimize surface area. For such a small droplet, surface tension will cause an increase of internal pressure p in order to balance the surface force.
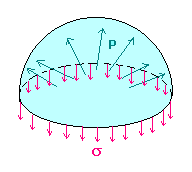 We will find the amount D (Dp = p - poutside) by which the pressure inside a liquid droplet of radius r, exceeds the pressure of the surrounding vapor/air by making force balances on a hemispherical drop. Observe that the internal pressure p is trying to blow apart the two hemispheres, whereas the surface tension s is trying to pull them together. Therefore, Dp pr2 = 2prs
We will find the amount D (Dp = p - poutside) by which the pressure inside a liquid droplet of radius r, exceeds the pressure of the surrounding vapor/air by making force balances on a hemispherical drop. Observe that the internal pressure p is trying to blow apart the two hemispheres, whereas the surface tension s is trying to pull them together. Therefore, Dp pr2 = 2prsi.e. Dp = 2s/r
Similar force balances can be made for cylindrical liquid jet.
Dp 2r= 2s
i.e. Dp = s/r
 Similar treatment can be made for a soap bubble which is having two free surfaces. Dp pr2 = 2 x 2prs
Similar treatment can be made for a soap bubble which is having two free surfaces. Dp pr2 = 2 x 2prsi.e. Dp = 4s/r
Surface tension generally appears only in situations involving either free surfaces (liquid/gas or liquid/solid boundaries) or interfaces (liquid/liquid boundaries); in the latter case, it is usually called the interfacial tension.
Representative values for the surface tensions of liquids at 20oC, in contact either with air or their vapor (there is usually little difference between the two), are given in Table.
| Liquid | Surface Tension s dyne/cm |
| Benzene | 23.70 |
| Benzene | 28.85 |
| Ethanol | 22.75 |
| Glycerol | 63.40 |
| Mercury | 435.50 |
| Methanol | 22.61 |
| n-Octane | 21.78 |
| Water | 72.75 |
Rise or fall of a liquid in a capillary tube is caused by surface tension and depends on the relative magnitude of cohesion of the liquid and the adhesion of the liquid to the walls of the containing vessel.
Liquids rise in tubes if they wet (adhesion > cohesion) and fall in tubes that do not wet (cohesion > adhesion).
Wetting and contact angle
Fluids wet some solids and do not others.
The figure shows some of the possible wetting behaviors of a drop of liquid placed on a horizontal, solid surface (the remainder of the surface is covered with air, so two fluids are present).
Fig.(a) represents the case of a liquid which wets a solid surface well, e.g. water on a very clean copper. The angle q shown is the angle between the edge of the liquid surface and the solid surface, measured inside the liquid. This angle is called the contact angle and is a measure of the quality of wetting. For perfectly wetting, in which the liquid spreads as a thin film over the surface of the solid, q is zero.
Fig.(c) represents the case of no wetting. If there were exactly zero wetting, q would be 180o. However, the gravity force on the drop flattens the drop, so that 180o angle is never observed. This might represent water on teflon or mercury on clean glass.
We normally say that a liquid wets a surface if q is less than 90o and does not wet if q is more than 90o. Values of q less than 20o are considered strong wetting, and values of q greater than 140o are strong nonwetting.
Capillarity is important (in fluid measurments) when using tubes smaller than about 10 mm in diameter.
Capillary rise (or depression) in a tube can be calculated by making force balances. The forces acting are force due to surface tension and gravity.
The force due to surface tesnion,
Fs = pdscos(q), where q is the wetting angle or contact angle. If tube (made of glass) is clean q is zero for water and about 140o for Mercury.
This is opposed by the gravity force on the column of fluid, which is equal to the height of the liquid which is above (or below) the free surface and which equals
Fg = (p/4)d2hgr,
where r is the density of liquid.
Equating these forces and solving for Capillary rise (or depression), we find
h = 4scos(q)/(rgd)
Fluid statics:
Pascal's law for pressure at a point:
The basic property of a static fluid is pressure. Pressure is familiar as a surface force exerted by a fluid against the walls of its container. Pressure also exists at every point within a volume of fluid. For a static fluid, as shown by the following analysis, pressure turns to be independent direction.
By considering the equilibrium of a small fluid element in the form of a triangular prism ABCDEF surrounding a point in the fluid, a relationship can be established between the pressures Px in the x direction, Pyin the y direction, and Ps normal to any plane inclined at any angle q to the horizontal at this point.
Px is acting at right angle to ABEF, and Py at right angle to CDEF, similarly Ps at right angle to ABCD.
Since there can be no shearing forces for a fluid at rest, and there will be no accelerating forces, the sum of the forces in any direction must therefore, be zero. The forces acting are due to the pressures on the surrounding and the gravity force.
Force due to Px = Px x Area ABEF = Pxdydz
Horizontal component of force due to Ps = - (Ps x Area ABCD) sin(q) = - Psdsdz dy/ds = -Psdydz
As Py has no component in the x direction, the element will be in equilibrium, if
Pxdydz + (-Psdydz) = 0
i.e. Px = Ps
Similarly in the y direction, force due to Py = Pydxdz
Component of force due to Ps = - (Ps x Area ABCD) cos(q) = - Psdsdz dx/ds = - Psdxdz
Force due to weight of element = - mg = - rVg = - r (dxdydz/2) g
Since dx, dy, and dz are very small quantities, dxdydz is negligible in comparison with other two vertical force terms, and the equation reduces to,
Py = Ps
Therefore, Px = Py = Ps
i.e. pressure at a point is same in all directions. This is Pascal's law. This applies to fluid at rest.
Fine powdery solids resemble fluids in many respects but differs considerably in others. For one thing, a static mass of particulate solids, can support shear stresses of considerable magnitude and the pressure is not the same in all directions.
Variation of pressure with elevation:
Consider a hypothetical differential cylindrical element of fluid of cross sectional area A and height (z2 - z1).
Upward force due to pressure P1 on the element = P1A
Downward force due to pressure P2 on the element = P2A
Force due to weight of the element = mg = rA(z2 - z1)g
Equating the upward and downward forces,
P1A = P2A + rA(z2 - z1)g
P2 - P1 = - rg(z2 - z1)
Thus in any fluid under gravitational acceleration, pressure decreases, with increasing height z in the upward direction.
Equality of pressure at the same level in a static fluid:
Equating the horizontal forces, P1A = P2A (i.e. some of the horizontal forces must be zero)
Equality of pressure at the same level in a continuous body of fluid:
Pressures at the same level will be equal in a continuous body of fluid, even though there is no direct horizontal path between P and Q provided that P and Q are in the same continuous body of fluid.
We know that, PR = PS
PR = PP + rgh à 1
PS = PQ + rgh à 2
From equn.1 and 2, PP = PQ
General equation for the variation of pressure due to gravity from point to point in a static fluid:
Resolving the forces along the axis PQ,
pA - (p + dp)A - rgAds cos(q) = 0
dp = - rgds cos(q)
or in differential form,
dp/ds = - rgcos(q)
In the vertical z direction, q = 0.
Therefore,
dp/dz = -rg
This equation predicts a pressure decrease in the vertically upwards direction at a rate proportional to the local density.
Absolute Pressure, Gage Pressure, and Vacuum
In a region such as outer space, which is virtually void of gases, the pressure is essentially zero. Such a condition can be approached very nearly in a laboratory when a vacuum pump is used to evacuate a bottle. The pressure in a vacuum is called absolute zero, and all pressures refernced with respect to this zero pressure are termed absolute pressures.
Many pressure-measuring devices measure not absolute pressure but only difference in pressure. For example, a Bourdon-tube gage indicates only the difference between the pressure in the fluid to which it is tapped and the pressure in the atmosphere. In this case, then, the reference pressure is actually the atmospheric pressure. This type of pressure reading is called gage pressure. For example, if a pressure of 50 kPa is measured with a gage referenced to the atmosphere and the atmospheric pressure is 100 kPa, then the pressure can be expressed as either
p = 50 kPa gage or p = 150 kPa absolute.
Whenever atmospheric pressure is used as a reference, the possibility exists that the pressure thus measured can be either positive or negative. Negative gage pressure are also termed as vacuum pressures. Hence, if a gage tapped into a tank indicates a vacuum pressure of 31 kPa, this can also be stated as 70 kPa absolute, or -31 kPa gage, assuming that the atmospheric pressure is 101 kPa absolute.
Pressure Measurement
Fluid Pressure
In a stationary fluid the pressure is exerted equally in all directions and is referred to as the static pressure. In a moving fluid, the static pressure is exerted on any plane parallel to the direction of motion. The fluid pressure exerted on a plane right angles to the direction of flow is greater than the static pressure because the surface has, in addition, to exert sufficient force to bring the fluid to rest. The additional pressure is proportional to the kinetic energy of fluid; it cannot be measured independently of the static pressure.
When the static pressure in a moving fluid is to be determined, the measuring surface must be parallel to the direction of flow so that no kinetic energy is converted into pressure energy at the surface. If the fluid is flowing in a circular pipe the measuring surface must be perpendicular to the radial direction at any point. The pressure connection, which is known as a piezometer tube, should flush with the wall of the pipe so that the flow is not disturbed: the pressure is then measured near the walls were the velocity is a minimum and the reading would be subject only to a small error if the surface were not quite parallel to the direction of flow.
The static pressure should always be measured at a distance of not less than 50 diameters from bends or other obstructions, so that the flow lines are almost parallel to the walls of the tube. If there are likely to be large cross-currents or eddies, a piezometer ring should be used. This consists of 4 pressure tappings equally spaced at 90o intervals round the circumference of the tube; they are joined by a circular tube which is connected to the pressure measuring device. By this means, false readings due to irregular flow or avoided. If the pressure on one side of the tube is relatively high, the pressure on the opposite side is generally correspondingly low; with the piezometer ring a mean value is obtained.BarometersA barometer is a device for measuring atmospheric pressure. A simple barometer consists of a tube more than 30 inch (760 mm) long inserted in an open container of mercury with a closed and evacuated end at the top and open tube end at the bottom and with mercury extending from the container up into the tube. Strictly, the space above the liquid cannot be a true vaccum. It contains mercury vapor at its saturated vapor pressure, but this is extremely small at room temperatures (e.g. 0.173 Pa at 20oC).
The atmospheric pressure is calculated from the relation Patm = ρgh where ρ is the density of fluid in the barometer.
PiezometerFor measuring pressure inside a vessel or pipe in which liquid is there, a tube may be attached to the walls of the container (or pipe) in which the liquid resides so liquid can rise in the tube. By determining the height to which liquid rises and using the relation P1 = ρgh, gauge pressure of the liquid can be determined. Such a device is known as piezometer. To avoid capillary effects, a piezometer's tube should be about 1/2 inch or greater. It is important that the opening of the device to be tangential to any fluid motion, otherwise an erroneous reading will result.
Manometers:
Manometers
A somewhat more complicated device for measuring fluid pressure consists of a bent tube containing one or more liquid of different specific gravities. Such a device is known as manometer.
In using a manometer, generally a known pressure (which may be atmospheric) is applied to one end of the manometer tube and the unknown pressure to be determined is applied to the other end.
In some cases, however, the difference between pressure at ends of the manometer tube is desired rather than the actual pressure at the either end. A manometer to determine this differential pressure is known asdifferential pressure manometer.
Manometers - Various forms
- Simple U - tube Manometer
- Inverted U - tube Manometer
- U - tube with one leg enlarged
- Two fluid U - tube Manometer
- Inclined U - tube Manometer
Simple U - tube ManometerEquating the pressure at the level XX'(pressure at the same level in a continuous body of fluid is equal),
For the left hand side:
Px = P1 + rg(a+h)
 For the right hand side:
For the right hand side:
Px' = P2 + rga + rmgh
Since Px = Px'
P1 + rg(a+h) = P2 + rga + rmgh
P1 - P2 = rmgh - rgh
i.e. P1 - P2 = (rm - r)gh.
The maximum value of P1 - P2 is limited by the height of the manometer. To measure larger pressure differences we can choose a manometer with heigher density, and to measure smaller pressure differences with accuracy we can choose a manometer fluid which is having a density closer to the fluid density.
Inverted U - tube Manometer
Inverted U-tube manometer is used for measuring pressure differences in liquids. The space above the liquid in the manometer is filled with air which can be admitted or expelled through the tap on the top, in order to adjust the level of the liquid in the manometer.
Equating the pressure at the level XX'(pressure at the same level in a continuous body of static fluid is equal),
For the left hand side:
Px = P1 - rg(h+a)
 For the right hand side:
For the right hand side:
Px' = P2 - (rga + rmgh)
Since Px = Px'
P1 - rg(h+a) = P2 - (rga + rmgh)
P1 - P2 = (r - rm)gh
If the manometric fluid is choosen in such a way that rm << r then,
P1 - P2 = rgh.
For inverted U - tube manometer the manometric fluid is usually air.
U - tube with one leg enlarged
Industrially, the simple U - tube manometer has the disadvantage that the movement of the liquid in both the limbs must be read. By making the diameter of one leg large as compared with the other, it is possible to make the movement the large leg very small, so that it is only necessary to read the movement of the liquid in the narrow leg.
In figure, OO' represents the level of liquid surface when the pressure difference P1 - P2 is zero. Then when pressure is applied, the level in the right hand limb will rise a distance h vertically.
Volume of liquid transferred from left-hand leg to right-hand leg
= h(p/4)d2
where d is the diameter of smaller diameter leg. If D is the diameter of larger diameter leg, then, fall in level of left-hand leg
= Volume transferred/Area of left-hand leg
= (h(p/4)d2) / ((p/4)D2)
= h(d/D)2
For the left-hand leg, pressure at X , i.e. Px = P1 + rg(h+a) + rg h(d/D)2
For the right-hand leg, pressure at X', i.e. Px' = P2 + rga + rg(h + h(d/D)2)
For the equality of pressure at XX',
P1 + rg(h+a) + rg h(d/D)2 = P2 + rga + rmg(h + h(d/D)2)
P1 - P2 = rmg(h + h(d/D)2) - rgh - rg h(d/D)2
If D>>d then, the term h(d/D)2 will be negligible( i.e approximately about zero)
Then P1 - P2 = (rm - r)gh.
Where h is the manometer liquid rise in the right-hand leg.
If the fluid density is negligible compared with the manometric fluid density ( eg. the case for air as the fluid and water as manometric fluid ), then P1 - P2 = rm gh.
Manometers Limitations:
The manometer in its various forms is an extremely useful type of pressure measuring instrument, but suffers from a number of limitations.
For the left hand side:
Px = P1 + rg(a+h)
 For the right hand side:
For the right hand side:Px' = P2 + rga + rmgh
Since Px = Px'
P1 + rg(a+h) = P2 + rga + rmgh
P1 - P2 = rmgh - rgh
i.e. P1 - P2 = (rm - r)gh.
The maximum value of P1 - P2 is limited by the height of the manometer. To measure larger pressure differences we can choose a manometer with heigher density, and to measure smaller pressure differences with accuracy we can choose a manometer fluid which is having a density closer to the fluid density.
Inverted U - tube Manometer
Inverted U-tube manometer is used for measuring pressure differences in liquids. The space above the liquid in the manometer is filled with air which can be admitted or expelled through the tap on the top, in order to adjust the level of the liquid in the manometer.
Equating the pressure at the level XX'(pressure at the same level in a continuous body of static fluid is equal),
For the left hand side:
Px = P1 - rg(h+a)
 For the right hand side:
For the right hand side:Px' = P2 - (rga + rmgh)
Since Px = Px'
P1 - rg(h+a) = P2 - (rga + rmgh)
P1 - P2 = (r - rm)gh
If the manometric fluid is choosen in such a way that rm << r then,
P1 - P2 = rgh.
For inverted U - tube manometer the manometric fluid is usually air.
U - tube with one leg enlarged
Industrially, the simple U - tube manometer has the disadvantage that the movement of the liquid in both the limbs must be read. By making the diameter of one leg large as compared with the other, it is possible to make the movement the large leg very small, so that it is only necessary to read the movement of the liquid in the narrow leg.
In figure, OO' represents the level of liquid surface when the pressure difference P1 - P2 is zero. Then when pressure is applied, the level in the right hand limb will rise a distance h vertically.
Volume of liquid transferred from left-hand leg to right-hand leg
= h(p/4)d2
where d is the diameter of smaller diameter leg. If D is the diameter of larger diameter leg, then, fall in level of left-hand leg
= Volume transferred/Area of left-hand leg
= (h(p/4)d2) / ((p/4)D2)
= h(d/D)2
For the left-hand leg, pressure at X , i.e. Px = P1 + rg(h+a) + rg h(d/D)2
For the right-hand leg, pressure at X', i.e. Px' = P2 + rga + rg(h + h(d/D)2)
For the equality of pressure at XX',
P1 + rg(h+a) + rg h(d/D)2 = P2 + rga + rmg(h + h(d/D)2)
P1 - P2 = rmg(h + h(d/D)2) - rgh - rg h(d/D)2
If D>>d then, the term h(d/D)2 will be negligible( i.e approximately about zero)
Then P1 - P2 = (rm - r)gh.
Where h is the manometer liquid rise in the right-hand leg.
If the fluid density is negligible compared with the manometric fluid density ( eg. the case for air as the fluid and water as manometric fluid ), then P1 - P2 = rm gh.
Manometers Limitations:
The manometer in its various forms is an extremely useful type of pressure measuring instrument, but suffers from a number of limitations.
- While it can be adapted to measure very small pressure differences, it can not be used conveniently for large pressure differences - although it is possible to connect a number of manometers in series and to use mercury as the manometric fluid to improve the range. (limitation)
- A manometer does not have to be calibrated against any standard; the pressure difference can be calculated from first principles. ( Advantage)
- Some liquids are unsuitable for use because they do not form well-defined menisci. Surface tension can also cause errors due to capillary rise; this can be avoided if the diameters of the tubes are sufficiently large - preferably not less than 15 mm diameter. (limitation)
- A major disadvantage of the manometer is its slow response, which makes it unsuitable for measuring fluctuating pressures.(limitation)
- It is essential that the pipes connecting the manometer to the pipe or vessel containing the liquid under pressure should be filled with this liquid and there should be no air bubbles in the liquid.(important point to be kept in mind)
Pressure GaugesBourdon Gauge:
The pressure to be measured is applied to a curved tube, oval in cross section. Pressure applied to the tube tends to cause the tube to straighten out, and the deflection of the end of the tube is communicated through a system of levers to a recording needle. This gauge is widely used for steam and compressed gases. The pressure indicated is the difference between that communicated by the system to the external (ambient) pressure, and is usually referred to as the gauge pressure.
Buoyancy
Upthrust on body = weight of fluid displaced by the body
This is known as Archimedes principle.
If the body is immersed so that part of its volume V1 is immersed in a fluid of density r1 and the rest of its volume V2 in another immiscible fluid of mass density r2,
Upthrust on upper part, R1 = r1gV1
acting through G1, the centroid of V1,
Upthrust on lower part,R2 = r2gV2
acting through G2, the centroid of V2,
Total upthrust = r1gV1 + r2gV2.
The positions of G1 and G2 are not necessarily on the same vertical line, and the centre of buoyancy of the whole body is, therefore, not bound to pass through the centroid of the whole body.
Systems of Units:
The official international system of units (System International d'Units). Strong efforts are underway for its universal adoption as the exclusive system for all engineering and science, but older systems, particularly the cgs and fps engineering gravitational systems are still in use and probably will be around for some time. The chemical engineer finds many physiochemical data given in cgs units; that many calculations are most conveniently made in fps units; and that SI units are increasingly encountered in science and engineering. Thus it becomes necessary to be expert in the use of all three systems.
SI system:Primary quantities:
Derived quantities:
cgs Units:The older centimeter-gram-second (cgs) system has the following units for derived quantities:
fps Units:The foot-bound-second (fps) system has long been used in commerce and engineering in English-speaking countries.
Conversion factors:Mass:1 lb = 0.454 kg
Length:1 inch = 2.54 cm = 0.0254 m
1 ft = 12 inch = 0.3048 m
Energy:1 BTU = 1055 J
1 cal = 4.184 J
Force:1 kgf = 9.812 N
1 lbf = 4.448 N
1 dyn = 1 g.cm/s2
Power:1 HP = 736 W
Pressure:1 Pa = 1 N/m2
1 psi = 1 lbf/inch2
1 atm = 1.01325 x 105 N/m2 = 14.7 psi
1 Bar = 105 N/m2
Viscosity:1 poise = 1 g/(cm.s)
1 cP = (1/100) poise = 0.001 kg/(m.s)
Kinematic viscosity:1 Stoke = 1 St = 1 cm2/s
Volume:1 ft3 = 7.481 U.S. gal
1 U.S. gal = 3.785 litre
Temperature:ToF = 32 + 1.8oC
ToR = 1.8K
Gas Constant:
R = 8314 J / (kmol.K)
Dimensions:
Dimensions of the primary quantities:
Dimensions of derived quantities, can be expressed in terms of the fundamental dimensions.
Similitude and model studies:
Whenever it is necessary to perform tests on a model to obtain information that cannot be obtained by analytical means alone, the rules of similitude must be applied. Similitude is the theory and art of predicting prototype performance from model observations.
Model Study: Present engineering practice makes use of model tests more frequently than most people realize. For example, whenever a new airplane is designed, tests are made not only on the general scale model but also on various components of the plane. Numerous tests are made on individual wing sections as well as on the engine pods and tail sections.
Models of automobiles and high-speed trains are also tested in wind tunnels to predict the drag and flow patterns for the prototype. Information derived from these model studies often indicates potential problems that can be corrected before prototype is built, thereby saving considerable time and expense in development of the prototype.
Marine engineers make extensive tests on model shop hulls to predict the drag of the ships.
Geometric similarity refers to linear dimensions. Two vessels of different sizes are geometrically similar if the ratios of the corresponding dimensions on the two scales are the same. If photographs of two vessels are completely super-impossible, they are geometrically similar.
Kinematic similarity refers to motion and requires geometric similarity and the same ratio of velocities for the corresponding positions in the vessels.
Dynamic similarity concerns forces and requires all force ratios for corresponding positions to be equal in kinematically similar vessels.
The requirement for similitude of flow between model and prototype is that the significant dimensionless parameters must be equal for model and prototype
Dimensional Analysis:
Many important engineering problems cannot be solved completely by theoretical or mathematical methods. Problems of this type are especially common in fluid-flow, heat-flow, and diffusional operations. One method of attacking a problem for which no mathematical equation can be derived is that of empirical experimentations. For example, the pressure loss from friction in a long, round, straight, smooth pipe depends on all these variables: the length and diameter of the pipe, the flow rate of the liquid, and the density and viscosity of the liquid. If any one of these variables is changed, the pressure drop also changes. The empirical method of obtaining an equation relating these factors to pressure drop requires that the effect of each separate variable be determined in turn by systematically varying that variable while keep all others constant. The procedure is laborious, and is difficult to organize or correlate the results so obtained into a useful relationship for calculations.
There exists a method intermediate between formal mathematical development and a completely empirical study. It is based on the fact that if a theoretical equation does exist among the variables affecting a physical process, that equation must be dimensionally homogeneous. Because of this requirement it is possible to group many factors into a smaller number of dimensionless groups of variables. The groups themselves rather than the separate factors appear in the final equation.
Dimensional analysis does not yield a numerical equation, and experiment is required to complete the solution of the problem. The result of a dimensional analysis is valuable in pointing a way to correlations of experimental data suitable for engineering use.
Dimensional analysis drastically simplifies the task of fitting experimental data to design equations where a completely mathematical treatment is not possible; it is also useful in checking the consistency of the units in equations, in converting units, and in the scale-up of data obtained in physical models to predict the performance of full-scale model. The method is based on the concept of dimension and the use ofdimensional formulas.
Important Dimensionless Numbers in Fluid Mechanics:
The pressure to be measured is applied to a curved tube, oval in cross section. Pressure applied to the tube tends to cause the tube to straighten out, and the deflection of the end of the tube is communicated through a system of levers to a recording needle. This gauge is widely used for steam and compressed gases. The pressure indicated is the difference between that communicated by the system to the external (ambient) pressure, and is usually referred to as the gauge pressure.
Buoyancy
Upthrust on body = weight of fluid displaced by the body
This is known as Archimedes principle.
If the body is immersed so that part of its volume V1 is immersed in a fluid of density r1 and the rest of its volume V2 in another immiscible fluid of mass density r2,
Upthrust on upper part, R1 = r1gV1
acting through G1, the centroid of V1,
Upthrust on lower part,R2 = r2gV2
acting through G2, the centroid of V2,
Total upthrust = r1gV1 + r2gV2.
The positions of G1 and G2 are not necessarily on the same vertical line, and the centre of buoyancy of the whole body is, therefore, not bound to pass through the centroid of the whole body.
Systems of Units:
The official international system of units (System International d'Units). Strong efforts are underway for its universal adoption as the exclusive system for all engineering and science, but older systems, particularly the cgs and fps engineering gravitational systems are still in use and probably will be around for some time. The chemical engineer finds many physiochemical data given in cgs units; that many calculations are most conveniently made in fps units; and that SI units are increasingly encountered in science and engineering. Thus it becomes necessary to be expert in the use of all three systems.
SI system:Primary quantities:
| Quantity | Unit |
| Mass in Kilogram | kg |
| Length in Meter | m |
| Time in Second | s or as sec |
| Temperature in Kelvin | K |
| Mole | gmol or simply as mol |
| Quantity | Unit |
| Force in Newton (1 N = 1 kg.m/s2) | N |
| Pressure in Pascal (1 Pa = 1 N/m2) | N/m2 |
| Work, energy in Joule ( 1 J = 1 N.m) | J |
| Power in Watt (1 W = 1 J/s) | W |
| Quantity | Unit |
| Force in dyne (1 dyn = 1 g.cm/s2) | dyn |
| Work, energy in erg ( 1 erg = 1 dyn.cm = 1 x 10-7 J ) | erg |
| Heat Energy in calorie ( 1 cal = 4.184 J) | cal |
| Quantity | Unit |
| Mass in pound ( 1 lb = 0.454 kg) | lb |
| Length in foot (1 ft = 0.3048 m) | ft |
| Temperature in Rankine | oR |
| Force in lbf ( 1 lbf = 32.2 lb.ft/s2) | lbf |
Length:1 inch = 2.54 cm = 0.0254 m
1 ft = 12 inch = 0.3048 m
Energy:1 BTU = 1055 J
1 cal = 4.184 J
Force:1 kgf = 9.812 N
1 lbf = 4.448 N
1 dyn = 1 g.cm/s2
Power:1 HP = 736 W
Pressure:1 Pa = 1 N/m2
1 psi = 1 lbf/inch2
1 atm = 1.01325 x 105 N/m2 = 14.7 psi
1 Bar = 105 N/m2
Viscosity:1 poise = 1 g/(cm.s)
1 cP = (1/100) poise = 0.001 kg/(m.s)
Kinematic viscosity:1 Stoke = 1 St = 1 cm2/s
Volume:1 ft3 = 7.481 U.S. gal
1 U.S. gal = 3.785 litre
Temperature:ToF = 32 + 1.8oC
ToR = 1.8K
Gas Constant:
R = 8314 J / (kmol.K)
Dimensions:
Dimensions of the primary quantities:
| Fundamental dimension | Symbol |
| Length | L |
| Mass | M |
| Time | t |
| Temperature | T |
| Quantity | Representative symbol | Dimensions |
| Angular velocity | w | t-1 |
| Area | A | L2 |
| Density | r | M/L3 |
| Force | F | ML/t2 |
| Kinematic viscosity | n | L2/t |
| Linear velocity | v | L/t |
| Linear acceleration | a | L/t2 |
| Mass flow rate | m. | M/t |
| Power | P | ML2/t3 |
| Pressure | p | M/Lt2 |
| Sonic velocity | c | L/t |
| Shear stress | t | M/Lt2 |
| Surface tension | s | M/t2 |
| Viscosity | m | M/Lt |
| Volume | V | L3 |
Similitude and model studies:
Model Study: Present engineering practice makes use of model tests more frequently than most people realize. For example, whenever a new airplane is designed, tests are made not only on the general scale model but also on various components of the plane. Numerous tests are made on individual wing sections as well as on the engine pods and tail sections.
Models of automobiles and high-speed trains are also tested in wind tunnels to predict the drag and flow patterns for the prototype. Information derived from these model studies often indicates potential problems that can be corrected before prototype is built, thereby saving considerable time and expense in development of the prototype.
Marine engineers make extensive tests on model shop hulls to predict the drag of the ships.
Geometric similarity refers to linear dimensions. Two vessels of different sizes are geometrically similar if the ratios of the corresponding dimensions on the two scales are the same. If photographs of two vessels are completely super-impossible, they are geometrically similar.
Kinematic similarity refers to motion and requires geometric similarity and the same ratio of velocities for the corresponding positions in the vessels.
Dynamic similarity concerns forces and requires all force ratios for corresponding positions to be equal in kinematically similar vessels.
The requirement for similitude of flow between model and prototype is that the significant dimensionless parameters must be equal for model and prototype
Dimensional Analysis:
Many important engineering problems cannot be solved completely by theoretical or mathematical methods. Problems of this type are especially common in fluid-flow, heat-flow, and diffusional operations. One method of attacking a problem for which no mathematical equation can be derived is that of empirical experimentations. For example, the pressure loss from friction in a long, round, straight, smooth pipe depends on all these variables: the length and diameter of the pipe, the flow rate of the liquid, and the density and viscosity of the liquid. If any one of these variables is changed, the pressure drop also changes. The empirical method of obtaining an equation relating these factors to pressure drop requires that the effect of each separate variable be determined in turn by systematically varying that variable while keep all others constant. The procedure is laborious, and is difficult to organize or correlate the results so obtained into a useful relationship for calculations.
There exists a method intermediate between formal mathematical development and a completely empirical study. It is based on the fact that if a theoretical equation does exist among the variables affecting a physical process, that equation must be dimensionally homogeneous. Because of this requirement it is possible to group many factors into a smaller number of dimensionless groups of variables. The groups themselves rather than the separate factors appear in the final equation.
Dimensional analysis does not yield a numerical equation, and experiment is required to complete the solution of the problem. The result of a dimensional analysis is valuable in pointing a way to correlations of experimental data suitable for engineering use.
Dimensional analysis drastically simplifies the task of fitting experimental data to design equations where a completely mathematical treatment is not possible; it is also useful in checking the consistency of the units in equations, in converting units, and in the scale-up of data obtained in physical models to predict the performance of full-scale model. The method is based on the concept of dimension and the use ofdimensional formulas.
Important Dimensionless Numbers in Fluid Mechanics:
| Dimensionless Number | Symbol | Formula | Numerator | Denominator | Importance |
| Reynolds number | NRe | Dvr/m | Inertial force | Viscous force | Fluid flow involving viscous and inertial forces |
| Froude number | NFr | u2/gD | Inertial force | Gravitational force | Fluid flow with free surface |
| Weber number | NWe | u2rD/s | Inertial force | Surface force | Fluid flow with interfacial forces |
| Mach number | NMa | u/c | Local velocity | Sonic velocity | Gas flow at high velocity |
| Drag coefficient | CD | FD/(ru2/2) | Total drag force | Inertial force | Flow around solid bodies |
| Friction factor | f | tw/(ru2/2) | Shear force | Inertial force | Flow though closed conduits |
| Pressure coefficient | CP | Dp/(ru2/2) | Pressure force | Inertial force | Flow though closed conduits. Pressure drop estimation |
No comments:
Post a Comment