The chemistry of life

In
this lens I'd like to talk you about the Chemistry of daily life.
Chemistry is an exciting experimental science which lets us to
understand our world and makes our life easier. As you will read in the
following articles, chemistry is in our everyday life: in our body, at
home, in the nature... in every second of our lives!
If any of you have a question about the chemistry of your everyday life, please feel free to contact me,
and I will publish here the answers to those that may be interesting
for most of the people. I invite you also to leave a comment in my
guestbook below.
Thank you very much to the
Squidoo staff for selecting this lens on April 28th as the 'lens of the
day', I feel honoured! I invite you also to visit my other lens: Spain in depth, and my website. Thank you!
Greetings from Spain,Silvia
How soap cleans?

There
are substances which can be dissolved in water (salt for example), and
others that can't (for example oil). Water and oil don't mix together,
so if we try to clean an oily stain from a cloth or from the skin, water
is not enough. We need soap.
Soap is formed by molecules with a "head" which likes water (hydrophilic) and a long chain which hates it (hydrophobic).
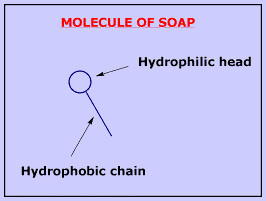
Because of this dualism, soap molecules act like a diplomat, improving the relationship between water and oil. How? When soap is added to the water, the hydrophilic heads of its molecules stay into the water (they like it!), while the long hydrophobic chains join the oil particles and remain inwards (escaping from the water). In that way, they form circular groups named micellas, with the oily material absorbed inside and trapped.
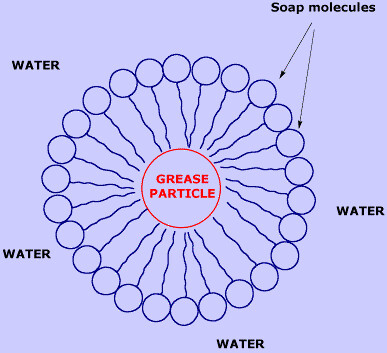
An emulsion of oil in water is then formed, this means that the oil particles become suspended and dispersed into the water. Thus, those oil particles are liberated from the cloth or the skin, and the emulsion is taken away with the rinsing.
In summary, soap cleans by acting as an emulsifier. It allows oil and water to mix so that oily grime can be removed during rinsing.There are more things involved in this process, such as for instance changes in the superficial tension of water, but this is the general idea.
Soap is formed by molecules with a "head" which likes water (hydrophilic) and a long chain which hates it (hydrophobic).

Because of this dualism, soap molecules act like a diplomat, improving the relationship between water and oil. How? When soap is added to the water, the hydrophilic heads of its molecules stay into the water (they like it!), while the long hydrophobic chains join the oil particles and remain inwards (escaping from the water). In that way, they form circular groups named micellas, with the oily material absorbed inside and trapped.

An emulsion of oil in water is then formed, this means that the oil particles become suspended and dispersed into the water. Thus, those oil particles are liberated from the cloth or the skin, and the emulsion is taken away with the rinsing.
In summary, soap cleans by acting as an emulsifier. It allows oil and water to mix so that oily grime can be removed during rinsing.There are more things involved in this process, such as for instance changes in the superficial tension of water, but this is the general idea.
Vegetables and colours - Part 1

White
light from the sun contains all the wavelengths, but when it impacts on
an object some of its wavelenghts are absorbed and some reflected. An
object is coloured because of the light that it reflects. For example
red objects reflect 'red' light, which is light with a long wavelength.
Many vegetables and fruits are strongly coloured because they contain an
especial kind of chemical compounds named carotenoids. These compounds have an area called choromophore, which absorbs and gives off particular wavelengths of light, generating the colour that we then perceive.
The chromophore is formed by a sequence of linear carbon-carbon double bonds (represented as C=C), much stronger than simple bonds (represented as C-C), so the atoms remain closer to each other. In general, it's necessary at least seven linear conjugated double bonds for a carotenoid to produce a colour. Besides, the bigger the number of bonds conjugated, the bigger the wavelength of the light absorbed and also the more red the vegetable, as you can see in this picture of the light spectrum:

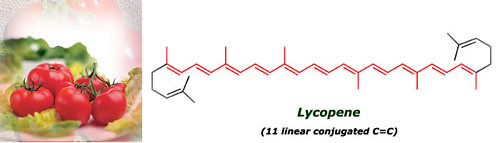
The tomato is red because of the carotenoid lycopene, which contains 11 conjugated carbon-carbon double bonds. You can count these bonds in the picture below, they are selected in red (the atom carbons are omitted, only the bonds are shown). This compound is generated by the plant to protect itself from the air oxidation. So it's a good antioxidant useful for us too, protecting our cells against the action of free radicals (potent oxidants), which are one of the main responsibles of cardiovascular diseases, cancer and aging.
The chromophore is formed by a sequence of linear carbon-carbon double bonds (represented as C=C), much stronger than simple bonds (represented as C-C), so the atoms remain closer to each other. In general, it's necessary at least seven linear conjugated double bonds for a carotenoid to produce a colour. Besides, the bigger the number of bonds conjugated, the bigger the wavelength of the light absorbed and also the more red the vegetable, as you can see in this picture of the light spectrum:

The tomato is red because of the carotenoid lycopene, which contains 11 conjugated carbon-carbon double bonds. You can count these bonds in the picture below, they are selected in red (the atom carbons are omitted, only the bonds are shown). This compound is generated by the plant to protect itself from the air oxidation. So it's a good antioxidant useful for us too, protecting our cells against the action of free radicals (potent oxidants), which are one of the main responsibles of cardiovascular diseases, cancer and aging.

(See the continuation in Part 2 below)
Vegetables and colour - Part 2
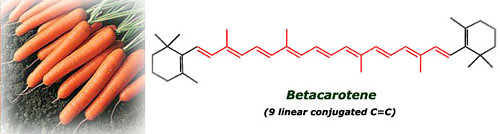
The pigment present in carrots is the betacarotene,
with 9 linear conjugated double bonds, less than in lycopene so they
are no red but orange (smaller wavelength than red, check it in the
spectrum picture). This compound is also a potent antioxidant and
besides it's transformed in our body into vitamin A, very important for
the maintenance of healthy skin, good vision and a robust inmune system.
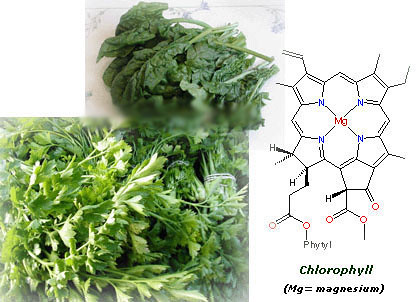
Spinachs, parsley and plants in general are green because they contain chlorophyll, a pigment which enables the plant to carry on photosynthesis, transforming solar energy and carbon dioxide into chemical energy in the form of carbohydrates and oxygen. This is a process essential for life.
As you can see in the pic below, the structure of chlorophyll is very complicated, so let's simpy say that it contains a big ring with a magnesium atom in the center. Curiously, the structure of hemoglobine (the carrier of oxygen in our blood) is pretty similar to chlorophyll, though it has an atom of iron instead of magnesium in its center.
The chlorophyll masks the other colours in vegetables and as its amount decreases the rest of colours become evident. This explains for example why tomatoes are initially green and then become red when they ripen.
This is an example of how Chemistry is everywhere, sometimes more evident, and sometimes much less :-).

Spinachs, parsley and plants in general are green because they contain chlorophyll, a pigment which enables the plant to carry on photosynthesis, transforming solar energy and carbon dioxide into chemical energy in the form of carbohydrates and oxygen. This is a process essential for life.
As you can see in the pic below, the structure of chlorophyll is very complicated, so let's simpy say that it contains a big ring with a magnesium atom in the center. Curiously, the structure of hemoglobine (the carrier of oxygen in our blood) is pretty similar to chlorophyll, though it has an atom of iron instead of magnesium in its center.
The chlorophyll masks the other colours in vegetables and as its amount decreases the rest of colours become evident. This explains for example why tomatoes are initially green and then become red when they ripen.

This is an example of how Chemistry is everywhere, sometimes more evident, and sometimes much less :-).
My guestbook
Please, leave your comments about this lens here
I invite you to leave your opinion about this lens in this space, clicking on the "Submit your own blurb" link below. You can also ask any question about chemistry of daily life.I'd love to read your comments! :-)
------------
Why meals are cooked faster in a pressure cooker?
 A
pressure cooker is like any other pot but with a more elaborated lid
that seals the pot completely. When you heat water inside the pot it
boils and the steam cannot escape, so it remains inside and starts to
build up pressure. Under pressure, cooking temperatures raise much
higher than under normal conditions (higher than the boiling point of
water, that is 100ºC), so then the food is cooked much faster. Cooking
times can be reduced by a factor of three or four.
A
pressure cooker is like any other pot but with a more elaborated lid
that seals the pot completely. When you heat water inside the pot it
boils and the steam cannot escape, so it remains inside and starts to
build up pressure. Under pressure, cooking temperatures raise much
higher than under normal conditions (higher than the boiling point of
water, that is 100ºC), so then the food is cooked much faster. Cooking
times can be reduced by a factor of three or four.Besides cooking faster, this method retains more nutrients present in the food than other methods. And did you know that a pressure cooker is often used by mountain climbers? Without it, water boils off before reaching 100ºC because of the lower atmospheric pressure at high altitudes, leaving the food improperly cooked.
Why do onions make you cry?

Who
has never cried while cutting an onion? (well, apart from those who
have never cut one hehehe). This is a little explanation in easy terms.
Inside the onion cells there are some chemical compounds that contain sulfur. When you cut an onion its cells are broken and those chemical compounds then undergo a reaction that transforms them into a more volatile sulfured products, which are released into the air.
These sulfured compounds react with the moisture in your eyes forming sulfuric acid, which produces a burning sensation. The nerve endings in your eyes are very sensitive and so they pick up on this irritation. The brain reacts by telling your tear ducts to produce more water, to dilute the irritating acid. So you cry to keep your eyes protected from the acid.
There are some tricks to make onion-dicing less problematic:
- Chop the onion under cold water. The volatile sulfured compounds will be released but then they react with the water, instead of reaching your eyes.
- You can freeze the onion for 10 minutes before cutting it. The cold temperature of the onion will slow down the chemical reaction which forms the volatile sulfured compunds.
Inside the onion cells there are some chemical compounds that contain sulfur. When you cut an onion its cells are broken and those chemical compounds then undergo a reaction that transforms them into a more volatile sulfured products, which are released into the air.
These sulfured compounds react with the moisture in your eyes forming sulfuric acid, which produces a burning sensation. The nerve endings in your eyes are very sensitive and so they pick up on this irritation. The brain reacts by telling your tear ducts to produce more water, to dilute the irritating acid. So you cry to keep your eyes protected from the acid.
There are some tricks to make onion-dicing less problematic:
- Chop the onion under cold water. The volatile sulfured compounds will be released but then they react with the water, instead of reaching your eyes.
- You can freeze the onion for 10 minutes before cutting it. The cold temperature of the onion will slow down the chemical reaction which forms the volatile sulfured compunds.
How to make invisible ink at home?
This is a funny experiment to do with kids at home.
 You
can play with your kids at home making invisible ink with basic
products that you have in the kitchen. There are many methods to make
invisible ink. These are some of the easiest ones:
You
can play with your kids at home making invisible ink with basic
products that you have in the kitchen. There are many methods to make
invisible ink. These are some of the easiest ones:1) Lemon juice method
- Ingredients: just lemon juice.
Write your message on a piece of paper with a brush or tootpick embedded in lemon juice. Let it dry completely.
To read the message heat the paper for a while (for instance hold it close to a light bulb) until the words become visible. Warning: do not hold paper too close to the heat and be careful not to let it get too hot!
- Chemical explanation: Lemon juice is a mild acid that weakens the paper upon contact. So when you heat the paper the part with the juice burns before the rest making your message visible.
2) Baking soda method
- Ingredients: baking soda and water in same amount (for instance 30 mL of each).
Mix them and use a toothpick or brush to write on a piece of paper. Wait until it dries completely.
To read the message paint the paper with a brush or a sponge embedded in concentrated grape juice. The message should show up.
- Chemical explanation: You've made an acid-base reaction. The baking soda is a basic compound that reacts with the acid contained in the apple juice, forming a new compound that has a different colour, making your message become visible.
A little poll
Would you like a world without chemistry?
What is cholesterol?
"Bad cholesterol" and "good cholesterol"... what's that?

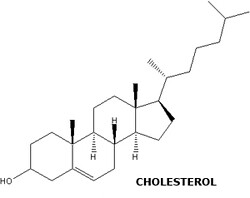
Cholesterol
is a fatty substance found in the blood of humans and also in the outer
lining of cells (membrane) in the body of animals. The cholesterol that
we have in our blood comes from two different sources:
- liver production
- diet: meat, fish, dairy products
After a meal, cholesterol is absorbed by the intestines, goes into the blood and then it's packeged inside a protein coat. These proteins are removed then by the liver.
When you go to the doctor, you are suggested to keep the "bad cholesterol" in blood low and the "good cholesterol" high. Ok. What does this mean?
- Bad cholesterol or LDL-low-density lipoprotein: These proteins deposit cholesterol on the artery walls, causing the formation of a hard substance named "cholesterol plaque". With the time, this plaque leds to narrowing of the arteries in a process called atherosclerosis. Because of this, the arteries can get blocked, so LDL is associated with a higher riks of coronary heart diseases.
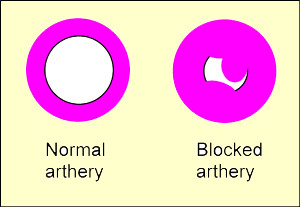
When the liver has many LDL receptors, this helps to remove more rapidly the LDL cholesterol from the blood, helping to keep the bad cholesterol levels low. The number of LDL receptors depends on both heredity and diet. For instance, people with familial hypercholesterolemia have a very low number of LDL receptors, so they usually have high levels of bad cholesterol. Besides, the bad cholesterol level can raise with diets high in saturated fats (certain vegetable oils and products derived mainly from meat and dairy products).
- Good cholesterol or HDL-high-density lipoprotein: These particles extract cholesterol from the artery walls and dispose them through the liver. So they prevent atherosclerosis.
Life style factors and other conditions influence HDL cholesterol levels. HDL cholesterol levels are for instance lower in smokers, people who eat a lot of sweets, and also in those who are overweight and inactive. On the other hand, estrogen increases the HDL cholesterol level, so usually women have higher good cholesterol levels than men.
These are some websites with interesting suggestions to keep your cholesterol at healthy levels:
- Low cholesterol diet.
- Cholesterol Lowdown.
- liver production
- diet: meat, fish, dairy products

After a meal, cholesterol is absorbed by the intestines, goes into the blood and then it's packeged inside a protein coat. These proteins are removed then by the liver.
When you go to the doctor, you are suggested to keep the "bad cholesterol" in blood low and the "good cholesterol" high. Ok. What does this mean?
- Bad cholesterol or LDL-low-density lipoprotein: These proteins deposit cholesterol on the artery walls, causing the formation of a hard substance named "cholesterol plaque". With the time, this plaque leds to narrowing of the arteries in a process called atherosclerosis. Because of this, the arteries can get blocked, so LDL is associated with a higher riks of coronary heart diseases.

When the liver has many LDL receptors, this helps to remove more rapidly the LDL cholesterol from the blood, helping to keep the bad cholesterol levels low. The number of LDL receptors depends on both heredity and diet. For instance, people with familial hypercholesterolemia have a very low number of LDL receptors, so they usually have high levels of bad cholesterol. Besides, the bad cholesterol level can raise with diets high in saturated fats (certain vegetable oils and products derived mainly from meat and dairy products).
- Good cholesterol or HDL-high-density lipoprotein: These particles extract cholesterol from the artery walls and dispose them through the liver. So they prevent atherosclerosis.
Life style factors and other conditions influence HDL cholesterol levels. HDL cholesterol levels are for instance lower in smokers, people who eat a lot of sweets, and also in those who are overweight and inactive. On the other hand, estrogen increases the HDL cholesterol level, so usually women have higher good cholesterol levels than men.
These are some websites with interesting suggestions to keep your cholesterol at healthy levels:
- Low cholesterol diet.
- Cholesterol Lowdown.
Why smoking is so harmful?

The
word "tobacco" is thought to derive from the Native American word
"tabago," for a Y-shaped pipe used in sniffing tobacco powder.
Cigarettes and other forms of tobacco consist of dried tobacco leaves,
and other ingredients added for flavor and other properties.
Some facts related with smoking:
- Smoking is the second major cause of death in the world. It's responsible for the death of one in ten adults worldwide.
- Smoking accounts for about 80-90% of all chronic obstructive pulmonary disease.
- Smoking is involved in 85% of all lung cancer deaths.
- Smoking is the major cause of cancer of the lips, tongue, mouth, pharynx, larynx and esophagus.
- Smoking has many other harmful effects in the body, a too long list to include it here.
Why smoking causes cancer? It's because tobacco and tobacco smoke contain more than 60 carcinogenic compounds. In general, more than 4,000 individual substances have been identified in tobacco smoke, including carbon monoxide, hydrogen cyanide, ammonia and other toxic irritants.
Besides all the harmful effects of tobacco, it's addictive, and this explains why although 70% of smokers want to quit and 35% attempt to quit each year, fewer than 7% succeed. The main reason why tobacco becomes addictive is due to its content of nicotine, which alters brain functioning.
Nicotine is a naturally occurring liquid alkaloid. An alkaloid is an organic compound made out of carbon, hydrogen, nitrogen and sometimes oxygen. These chemicals have potent effects on the human body. For example, many people enjoy the stimulating effects of another alkaloid, caffeine.
Some facts related with smoking:
- Smoking is the second major cause of death in the world. It's responsible for the death of one in ten adults worldwide.
- Smoking accounts for about 80-90% of all chronic obstructive pulmonary disease.
- Smoking is involved in 85% of all lung cancer deaths.
- Smoking is the major cause of cancer of the lips, tongue, mouth, pharynx, larynx and esophagus.
- Smoking has many other harmful effects in the body, a too long list to include it here.
Why smoking causes cancer? It's because tobacco and tobacco smoke contain more than 60 carcinogenic compounds. In general, more than 4,000 individual substances have been identified in tobacco smoke, including carbon monoxide, hydrogen cyanide, ammonia and other toxic irritants.
Besides all the harmful effects of tobacco, it's addictive, and this explains why although 70% of smokers want to quit and 35% attempt to quit each year, fewer than 7% succeed. The main reason why tobacco becomes addictive is due to its content of nicotine, which alters brain functioning.
Nicotine is a naturally occurring liquid alkaloid. An alkaloid is an organic compound made out of carbon, hydrogen, nitrogen and sometimes oxygen. These chemicals have potent effects on the human body. For example, many people enjoy the stimulating effects of another alkaloid, caffeine.
Nicotine:
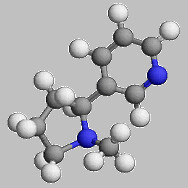

When you smoke, nicotine is absorbed through the skin
and mucosal lining of the mouth and nose or by inhalation in the lungs.
Once in the body, it activates the same reward system as do other drugs
such as cocaine or amphetamine, although to a lesser degree. In the
brain, nicotine increases the level of the neurotransmitter dopamine,
which is a chemical in the brain responsible for feelings of pleasure.
The acute effects of nicotine subside within minutes, so people continue
dosing themselves frequently throughout the day to maintain the
pleasurable effects of nicotine and to prevent withdrawal symptoms.
Some info about how to quit smoking:
http://www.cdc.gov/Tobacco/how2quit.htm
Some info about how to quit smoking:
http://www.cdc.gov/Tobacco/how2quit.htm
Why the sky is blue?
We all can look up to the sky and see its beautiful blue colour. Why is it blue, and not red, or white for instance?

As
I commented in the "Vegetables post", an object is coloured because of
the light that it reflects. The white light from the sun contains all
the wavelengths, but when it impacts on an object some of its
wavelengths are absorbed and some reflected. For example blue objects
reflect 'blue' light, which is light with a pretty short wavelength.
There is a phenomena named Rayleigh scattering, that consists on the scattering of light by particles much smaller than its wavelength. This effect is especially strong when light passes through gases.
Each of the wavelengths of light suffers a different scattering when it encounters the gas particles that form the atmosphere (nitrogen, oxygen...). This effect is more prominent in the case of short light wavelengths, that are the blue end of the visible spectrum, so the blue light becomes much more dispersed and it can be seen from every direction, as you can see in the drawing below. This gives us the impression that the sky is blue. On the other hand, the red colour is scattered much less, so it can be only seen from certain directions. In the drawing below, both Observer 1 and Observer 2 can see the blue light, but only Observer 2 is in the right direction to see the red one, and that's why we see those beautiful red skies at sunset sometimes.
There is a phenomena named Rayleigh scattering, that consists on the scattering of light by particles much smaller than its wavelength. This effect is especially strong when light passes through gases.
Each of the wavelengths of light suffers a different scattering when it encounters the gas particles that form the atmosphere (nitrogen, oxygen...). This effect is more prominent in the case of short light wavelengths, that are the blue end of the visible spectrum, so the blue light becomes much more dispersed and it can be seen from every direction, as you can see in the drawing below. This gives us the impression that the sky is blue. On the other hand, the red colour is scattered much less, so it can be only seen from certain directions. In the drawing below, both Observer 1 and Observer 2 can see the blue light, but only Observer 2 is in the right direction to see the red one, and that's why we see those beautiful red skies at sunset sometimes.
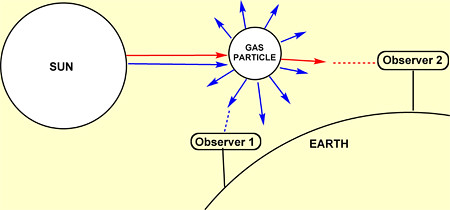
Then, why the clouds are white? Well, the water
droplets that form the clouds have a much larger size than the gas
particles of the air, and they scatter all the wavelenghts of light in
the same extent, so all of them are reflected equally and we receive
then the full colour of light, that is white.
Why coffee keeps you awake?

It is well-known that the effect of coffee on mood is related to its content in caffeine (you can see its pic on the right)
But why caffeine has such a strong effect on us? Caffeine operates using the same mechanisms of amphetamines, cocaine, and heroin to stimulate the brain, though with milder effects. It manipulates the same channels as the other drugs, and that is one of the things that gives caffeine its addictive qualities.
There is a chemical in our brain called adenosine, that binds to certain receptors and slows down nerve cell activity when we are sleeping. To a nerve cell, caffeine looks like adenosine and it binds to the adenosine receptors. However, as it's not really adenosine, it doesn't slow down the cell's activity like adenosine would. So the cell cannot "see" adenosine anymore because caffeine has taken up all the receptors adenosine binds to. Then, instead of slowing down because of the adenosine level, the cells speed up.
The pituitary gland sees all of this activity and thinks some sort of emergency must be occurring, so it releases hormones that tell the adrenal glands to produce adrenaline. Adrenaline is the "fight" hormone, and it makes your heart to beat faster, the breathing tubes to open up, the liver to release sugar into the bloodstream for extra energy and your muscles to tighten up, ready for action. Because of this, after consuming a big cup of coffee your muscles tense up, you feel excited and you can feel your heart beat increasing. Moreover, as amphetamines, caffeine also increases the levels of dopamine, which is associated with the pleasure system of the brain, providing feelings of enjoyment and reinforcement.
But why caffeine has such a strong effect on us? Caffeine operates using the same mechanisms of amphetamines, cocaine, and heroin to stimulate the brain, though with milder effects. It manipulates the same channels as the other drugs, and that is one of the things that gives caffeine its addictive qualities.
There is a chemical in our brain called adenosine, that binds to certain receptors and slows down nerve cell activity when we are sleeping. To a nerve cell, caffeine looks like adenosine and it binds to the adenosine receptors. However, as it's not really adenosine, it doesn't slow down the cell's activity like adenosine would. So the cell cannot "see" adenosine anymore because caffeine has taken up all the receptors adenosine binds to. Then, instead of slowing down because of the adenosine level, the cells speed up.
The pituitary gland sees all of this activity and thinks some sort of emergency must be occurring, so it releases hormones that tell the adrenal glands to produce adrenaline. Adrenaline is the "fight" hormone, and it makes your heart to beat faster, the breathing tubes to open up, the liver to release sugar into the bloodstream for extra energy and your muscles to tighten up, ready for action. Because of this, after consuming a big cup of coffee your muscles tense up, you feel excited and you can feel your heart beat increasing. Moreover, as amphetamines, caffeine also increases the levels of dopamine, which is associated with the pleasure system of the brain, providing feelings of enjoyment and reinforcement.
The Chemistry of Love - Part 1
Is there real chemistry behind love? This article is divided in two parts.

Answering
the question of last week, yes, there's a lot of real chemistry behind
love! Chemistry is in the roots of every step in a relationship, and
this field is under continuous research. When you fall in love, your
brain suffers some changes and also certain chemical compounds are
released. Researchers consider three stages in love: lust, attraction
and attachment, each of them involve different chemicals (don't worry
about the chemical names, simply get the essence!):
1) Lust
Lust is driven by initial physical attraction and flirting. This stage can depend on characteristics such as a symmetrical face and proportionate body dimensions. Flirting can include gazing into the eyes, touching, and mirroring in body language. The two chemicals that surface during this stage are the sex hormones (testosterone and estrogen) and pheromones.
In the animal world, PHEROMONES are individual scent "prints" found in urine or sweat that dictate sexual behavior and attract the opposite sex. The existence of human pheromones was discovered in 1986, finding these chemicals in human sweat.
2) Falling in love - Attraction
When you fall in love you may have many physical symptoms: lose of appetite, can't sleep, can't concentrate, palms sweat, butterflies in stomach... This is due to surging brain chemicals called monoamines:
- DOPAMINE: it's commonly associated with the pleasure system of the brain, providing feelings of enjoyment and reinforcement to motivate us to do certain activities. It's released by naturally-rewarding experiences, such as sex or food. Some research studies show that when female rodents were injected dopamine in the presence of a male rodent, the female will pick him out of a crowd later.
- PHENYLETHYLAMINE: It's a natural amphetamine like the known drug and can cause the same stimulation effects. It contributes to that on-top-of-the-world feeling that attraction can bring, and gives you the energy to stay up day and night with a new love.
- SEROTONIN: it controls impulses, unruly passions, obsessive behavior, aiding the sense of "being in control".
- NOREPINEPHRINE is another neurotransmitter which induces euphoria in your brain, exciting the body by giving it a booster dose of natural adrenaline. This causes the heart to beat faster and blood pressure to rise. That's why you can experience a pounding heart or sweaty palms when you see someone you're attracted to.
1) Lust
Lust is driven by initial physical attraction and flirting. This stage can depend on characteristics such as a symmetrical face and proportionate body dimensions. Flirting can include gazing into the eyes, touching, and mirroring in body language. The two chemicals that surface during this stage are the sex hormones (testosterone and estrogen) and pheromones.
In the animal world, PHEROMONES are individual scent "prints" found in urine or sweat that dictate sexual behavior and attract the opposite sex. The existence of human pheromones was discovered in 1986, finding these chemicals in human sweat.
2) Falling in love - Attraction
When you fall in love you may have many physical symptoms: lose of appetite, can't sleep, can't concentrate, palms sweat, butterflies in stomach... This is due to surging brain chemicals called monoamines:
- DOPAMINE: it's commonly associated with the pleasure system of the brain, providing feelings of enjoyment and reinforcement to motivate us to do certain activities. It's released by naturally-rewarding experiences, such as sex or food. Some research studies show that when female rodents were injected dopamine in the presence of a male rodent, the female will pick him out of a crowd later.
- PHENYLETHYLAMINE: It's a natural amphetamine like the known drug and can cause the same stimulation effects. It contributes to that on-top-of-the-world feeling that attraction can bring, and gives you the energy to stay up day and night with a new love.
- SEROTONIN: it controls impulses, unruly passions, obsessive behavior, aiding the sense of "being in control".
- NOREPINEPHRINE is another neurotransmitter which induces euphoria in your brain, exciting the body by giving it a booster dose of natural adrenaline. This causes the heart to beat faster and blood pressure to rise. That's why you can experience a pounding heart or sweaty palms when you see someone you're attracted to.
The Chemistry of Love - Part 2
Continuation of Part 1

3) Attachment - Staying together
There is a sense of calm and stability that we feel with a long-term partner, a sort of bond that keeps couples together. This kind of love is driven these hormones:
- OXYTOCIN: it's sometimes known as "the cuddle chemical." It's the hormone best known for its role in inducing labor by stimulating contractions. But recently it has been observed that it may influence our ability to bond with others, as both genders release this hormone when touching and cuddling, with the oxytocin level peaking during orgasm.
- VASOPRESSIN: also called as "the monogamy chemical". Researchers have found that suppression of vasopressin can cause males to abandon their love nest and seek new mates.
- ENDORPHINS: they are biochemical compounds that enhance our immune system, block the lesion of blood vessel, have anti-aging, anti-stress and pain-relieving effect, and also help to improve your memory.
High levels of oxytocin and vasopressin may interfere with dopamine and norepinephrine pathways, which may explain why with the time attachment grows as mad passionate love fades.
Well, as you can see, there is real chemistry taking place in our body when we are in love! This doesn't mean that love is only chemistry, but at least now you can understand this feeling from a different point of view, don't you?
There is a sense of calm and stability that we feel with a long-term partner, a sort of bond that keeps couples together. This kind of love is driven these hormones:
- OXYTOCIN: it's sometimes known as "the cuddle chemical." It's the hormone best known for its role in inducing labor by stimulating contractions. But recently it has been observed that it may influence our ability to bond with others, as both genders release this hormone when touching and cuddling, with the oxytocin level peaking during orgasm.
- VASOPRESSIN: also called as "the monogamy chemical". Researchers have found that suppression of vasopressin can cause males to abandon their love nest and seek new mates.
- ENDORPHINS: they are biochemical compounds that enhance our immune system, block the lesion of blood vessel, have anti-aging, anti-stress and pain-relieving effect, and also help to improve your memory.
High levels of oxytocin and vasopressin may interfere with dopamine and norepinephrine pathways, which may explain why with the time attachment grows as mad passionate love fades.
Well, as you can see, there is real chemistry taking place in our body when we are in love! This doesn't mean that love is only chemistry, but at least now you can understand this feeling from a different point of view, don't you?
Chemistry joke of the week
A neutron walks into a bar, sits down and asks for a drink. Finishing, the neutron asks "How much?"
The bartender says, "For you, no charge."
[Explanation for non-chemists: a neutron is a particle that has no charge. Proton are those with positive charge, and electrons those with negative charge.]
Lactose intolerance

Some
people suffer nausea or diarrhea after drinking milk or milk
derivatives. The origin of this problem can be the difficulty to digest
lactose.
Lactose is the main complex sugar found in the milk. It's a pretty big compound formed by two smaller components: glucose and galactose. Such a big compound cannot get through the intestinal wall and into the bloodstream, so we need "something" to break it into smaller pieces. This "something" is an enzime named lactase. The more milk and milk products we consume, the more lactase we need.
Normally there's plenty of lactase in the digestive systems of infants and children, but the ability to produce lactase in big amounts decreases as we grow older, generating usually too little to handle more than one or two glasses of milk at a time. When this drop in lactase production falls below certain minimums the intolerance to lactose appears.
Without enough lactase in the digestive fluids, the lactose of milk and milk products isn't broken effectively, so lactose passes along the intestinal path to a region where it undergoes fermentation to gases such as carbon dioxide and hydrogen and to acid lactic, a bowel irritant. The combination easily produces gastric distress and diarrhea.
I don't think there's any way to increase the amount of lactase enzyme the body can make, but fortunately the symptoms can be controlled through diet. There are lactose-reduced milk products at most supermarkets and even lactase additives available from drug stores without a prescription.
Lactose is the main complex sugar found in the milk. It's a pretty big compound formed by two smaller components: glucose and galactose. Such a big compound cannot get through the intestinal wall and into the bloodstream, so we need "something" to break it into smaller pieces. This "something" is an enzime named lactase. The more milk and milk products we consume, the more lactase we need.
Normally there's plenty of lactase in the digestive systems of infants and children, but the ability to produce lactase in big amounts decreases as we grow older, generating usually too little to handle more than one or two glasses of milk at a time. When this drop in lactase production falls below certain minimums the intolerance to lactose appears.
Without enough lactase in the digestive fluids, the lactose of milk and milk products isn't broken effectively, so lactose passes along the intestinal path to a region where it undergoes fermentation to gases such as carbon dioxide and hydrogen and to acid lactic, a bowel irritant. The combination easily produces gastric distress and diarrhea.
I don't think there's any way to increase the amount of lactase enzyme the body can make, but fortunately the symptoms can be controlled through diet. There are lactose-reduced milk products at most supermarkets and even lactase additives available from drug stores without a prescription.
How is coffee decaffeinated?

There are different ways of removing caffeine from coffee:
- Water extraction: At the beginning solvents like dichloromethane or ethyl acetate were used because they dissolve selectively the caffeine. But because of their toxicity nowadays water is used instead. Hot water extracts both flavor ingredients and caffeine from green coffee beans. Then the extract is passed through activated charcoal and most of the caffeine is removed. Finally, soaking the original beans in the decaffeinated extract restores most of their flavor.
- Supercritical fluid CO2 extraction: Supercritical fluids have both gaslike and liquidlike properties, they fill the container like a gas but can dissolve substances like a liquid. In the caffeine extraction process, this fluid is forced through green coffee beans, it penetrates deep into the beans and dissolves most of the caffeine present. For more information about supercritical fluids click here.
- Genetic engineering: Some researchers are investigating ways to inactivate the gene that codes for caffeine synthasa, the enzyme which catalyzes the caffeine synthesis. Then coffee plants unable to produce caffeine could be grown.
- Water extraction: At the beginning solvents like dichloromethane or ethyl acetate were used because they dissolve selectively the caffeine. But because of their toxicity nowadays water is used instead. Hot water extracts both flavor ingredients and caffeine from green coffee beans. Then the extract is passed through activated charcoal and most of the caffeine is removed. Finally, soaking the original beans in the decaffeinated extract restores most of their flavor.
- Supercritical fluid CO2 extraction: Supercritical fluids have both gaslike and liquidlike properties, they fill the container like a gas but can dissolve substances like a liquid. In the caffeine extraction process, this fluid is forced through green coffee beans, it penetrates deep into the beans and dissolves most of the caffeine present. For more information about supercritical fluids click here.
- Genetic engineering: Some researchers are investigating ways to inactivate the gene that codes for caffeine synthasa, the enzyme which catalyzes the caffeine synthesis. Then coffee plants unable to produce caffeine could be grown.







No comments:
Post a Comment